Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
For optimal and reproducible results, BD Horizon Brilliant Stain Buffer should be used anytime two or more BD Horizon Brilliant dyes are used in the same experiment. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. The BD Horizon Brilliant Stain Buffer was designed to minimize these interactions. More information can be found in the Technical Data Sheet of the BD Horizon Brilliant Stain Buffer (Cat. No. 563794 or 566349).
When setting up compensation, it is recommended to compare spillover values obtained from cells and BD™ CompBeads to ensure that beads will provide sufficiently accurate spillover values.
For optimal results, it is recommended to perform two washes after staining with antibodies. Cells may be prepared, stained with antibodies and washed twice with wash buffer per established protocols for immunofluorescent staining prior to acquisition on a flow cytometer. Performing fewer than the recommended wash steps may lead to increased spread of the negative population.
Product Notices
- This antibody was developed for use in flow cytometry.
- The production process underwent stringent testing and validation to assure that it generates a high-quality conjugate with consistent performance and specific binding activity. However, verification testing has not been performed on all conjugate lots.
- Researchers should determine the optimal concentration of this reagent for their individual applications.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- BD Horizon Brilliant Stain Buffer is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,575,303; 8,354,239.
- BD Horizon Brilliant Blue 700 is covered by one or more of the following US patents: 8,455,613 and 8,575,303.
- Cy is a trademark of GE Healthcare.
Companion Products
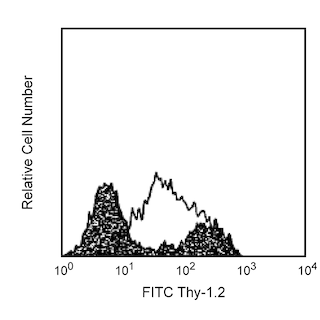



The RB40.34 monoclonal antibody specifically binds to mouse P-selectin (CD62P), a 140 kDa protein which is expressed on activated platelets, activated endothelial cells, and megakaryocytes. P-selectin mediates the adhesion of neutrophils and monocytes to activated platelets and endothelial cells, mediates leukocyte rolling, and is involved in the migration of leukocytes into inflamed tissues. CD24 and CD162 (PSGL-1) are ligands of CD62P. mAb RB40.34 can block mouse P-selectin binding to its ligands in vitro and in vivo.
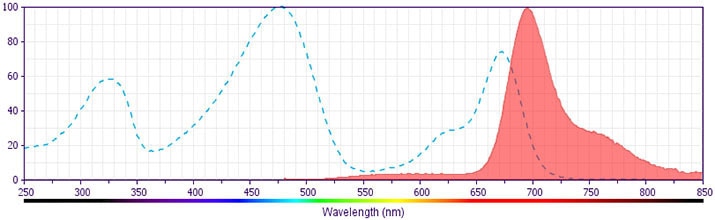
The antibody was conjugated to BD Horizon™ BB700, which is part of the BD Horizon Brilliant™ Blue family of dyes. It is a polymer-based tandem dye developed exclusively by BD Biosciences. With an excitation max of 485 nm and an emission max of 693 nm, BD Horizon BB700 can be excited by the 488 nm laser and detected in a standard PerCP-Cy™5.5 set (eg, 695/40-nm filter). This dye provides a much brighter alternative to PerCP-Cy5.5 with less cross laser excitation off the 405 nm and 355 nm lasers.
Development References (9)
-
Aigner S, Ruppert M, Hubbe M, et al. Heat stable antigen (mouse CD24) supports myeloid cell binding to endothelial and platelet P-selectin. Int Immunol. 1995; 7(10):1557-1565. (Biology). View Reference
-
Austrup F, Vestweber D, Borges E, et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997; 385(6611):81-83. (Clone-specific: Blocking). View Reference
-
Bosse R, Vestweber D. Only simultaneous blocking of the L- and P-selectin completely inhibits neutrophil migration into mouse peritoneum. Eur J Immunol. 1994; 24(12):3019-3024. (Immunogen: Blocking, ELISA, Flow cytometry, Immunoprecipitation). View Reference
-
Hirata T, Furie BC, Furie B. P-, E-, and L-selectin mediate migration of activated CD8+ T lymphocytes into inflamed skin. J Immunol. 2002; 169(8):4307-4313. (Clone-specific: Blocking). View Reference
-
Katakai T, Mori KJ, Masuda T, Shimizu A. Selective accumulation of type 1 effector cells expressing P-selectin ligand and/or alpha(4)beta(7)-integrin at the lesions of autoimmune gastritis. Int Immunol. 2002; 14(2):167-175. (Clone-specific: Immunohistochemistry). View Reference
-
Ley K, Bullard DC, Arbones ML, et al. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med. 1995; 181(2):669-675. (Clone-specific: Blocking). View Reference
-
Pendl GG, Robert C, Steinert M, et al. Immature mouse dendritic cells enter inflamed tissue, a process that requires E- and P-selectin, but not P-selectin glycoprotein ligand 1. Blood. 2002; 99(3):946-956. (Clone-specific: Blocking). View Reference
-
Tietz W, Allemand Y, Borges E, et al. CD4+ T cells migrate into inflamed skin only if they express ligands for E- and P-selectin. J Immunol. 1998; 161(2):963-970. (Clone-specific: Blocking). View Reference
-
Yang J, Galipeau J, Kozak CA, Furie BC, Furie B. Mouse P-selectin glycoprotein ligand-1: molecular cloning, chromosomal localization, and expression of a functional P-selectin receptor. Blood. 1996; 87(10):4176-4186. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.