-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- United Kingdom (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
BD Pharmingen™ Purified Mouse anti-α-Synuclein (pY125)
Clone I57-628 (RUO)
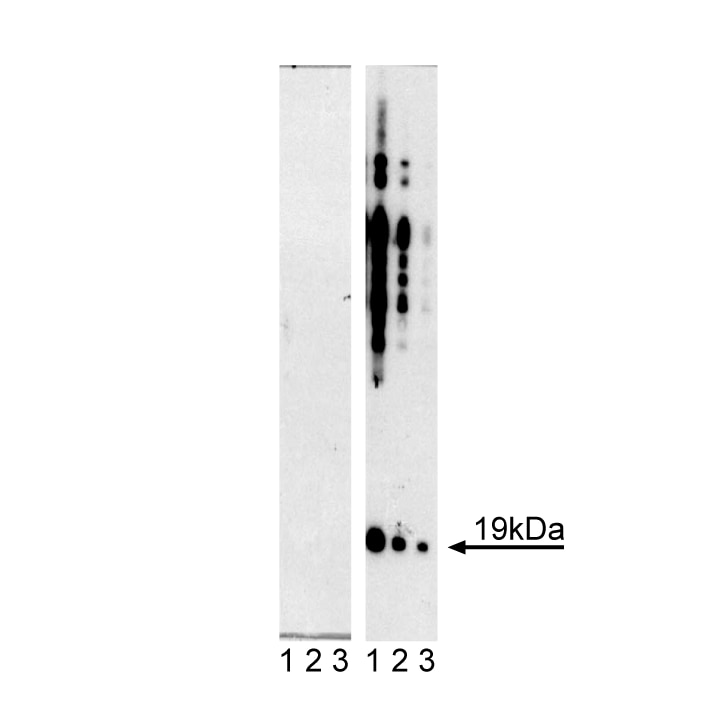
Western blot analysis of α-Synuclein (pY125). Lysates from control (left panel) and pervanadate-treated (right panel) HEK 293 cells were probed with mAb I57-628 at concentrations of 0.002, 0.001, and 0.0005 µg/ml (Lanes 1, 2, and 3, respectively). α-Synuclein (pY125) is identified as a strong band of 19 kDa in the pervanadate-treated
cells.


Western blot analysis of α-Synuclein (pY125). Lysates from control (left panel) and pervanadate-treated (right panel) HEK 293 cells were probed with mAb I57-628 at concentrations of 0.002, 0.001, and 0.0005 µg/ml (Lanes 1, 2, and 3, respectively). α-Synuclein (pY125) is identified as a strong band of 19 kDa in the pervanadate-treated
cells.

Western blot analysis of α-Synuclein (pY125). Lysates from control (left panel) and pervanadate-treated (right panel) HEK 293 cells were probed with mAb I57-628 at concentrations of 0.002, 0.001, and 0.0005 µg/ml (Lanes 1, 2, and 3, respectively). α-Synuclein (pY125) is identified as a strong band of 19 kDa in the pervanadate-treated
cells.


BD Pharmingen™ Purified Mouse anti-α-Synuclein (pY125)

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
Western blot: Please refer to http://www.bdbiosciences.com/pharmingen/protocols/Western_Blotting.shtml
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
The 140-amino-acid α-Synuclein protein is identical to the non-amyloid-β component precursor (NACP), a presynaptic protein involved in amyloidogenesis in Alzheimer's disease (AD). This protein is expressed in brain, primarily in presynaptic nerve terminals. Although the exact function of the Synucleins has not been determined, they have been linked to the prominent neurodegenerative disorders AD and Parkinson's disease. The Tyrosine 125 (Y125) residue of α-Synuclein plays an important role in stress-induced dimerization of the protein and is phosphorylated by Pyk/RAFTK via the Src-family kinases Fyn and c-Src.
The I57-628 antibody recognizes α-Synuclein phosphorylated at Y125.
Development References (5)
-
Ellis CE, Schwartzberg PL, Grider TL, Fink DW, Nussbaum RL. α-Synuclein is phosphorylated by members of the Src family of protein-tyrosine kinases. J Biol Chem. 2001; 276(6):3879-3884. (Biology).
-
Forman MS, Trojanowski JQ, Lee VM-Y. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004; 10(10):1055-1063. (Biology).
-
Nakamura T, Yamashita H, Nagano Y, et al. Activation of Pyk2/RAFTK induces tyrosine phosphorylation of α-synuclein via Src-family kinases. FEBS Lett. 2002; 521(1 - 3):190-194. (Biology).
-
Nakamura T, Yamashita H, Takahashi T, Nakamura S. Activated Fyn phosphorylates alpha-synuclein at tyrosine residue 125. Biochem Biophys Res Commun. 2001; 280(4):1085-1092. (Biology).
-
Takahashi T, Yamashita H, Nakamura T, Nagano Y, Nakamura S. Tyrosine 125 of alpha-synuclein plays a critical role for dimerization following nitrative stress. Brain Res. 2002; 938(1 - 2):73-80. (Biology).
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.