Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


.png)

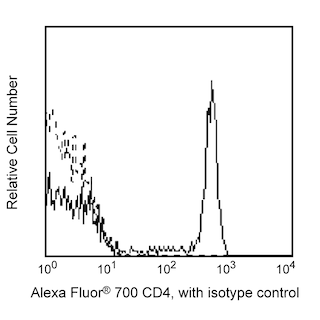
Multicolor flow cytometric analysis of FoxP3 expression in resting Human peripheral blood lymphocytes. Human peripheral blood mononuclear cells (PBMC) were stained with BD Pharmingen™ Alexa Fluor™ 700 Mouse Anti-Human CD4 antibody (Cat. No. 557922). The cells were fixed and permeabilized using the BD Pharmingen™ Transcription Factor Buffer Set (Cat. No. 562574/562725) followed by intracellular staining with either BD Horizon™ RB780 Mouse IgG1, κ Isotype Control (Cat. No. 568532; Left Plot) or BD Horizon™ RB780 Mouse Anti-Human FoxP3 antibody (Cat. No. 568682/568683; Right Plot). The two-parameter pseudocolor density plot showing the correlated expression of CD4 versus FoxP3 [or Ig Isotype control staining] was derived from gated events with the forward and side light-scatter characteristics of intact lymphocytes. Flow cytometry and data analysis were performed using a BD FACSymphony™ A5 Cell Analyzer System and FlowJo™ Software.
.png)

BD Horizon™ RB780 Mouse Anti-Human FoxP3
.png)
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
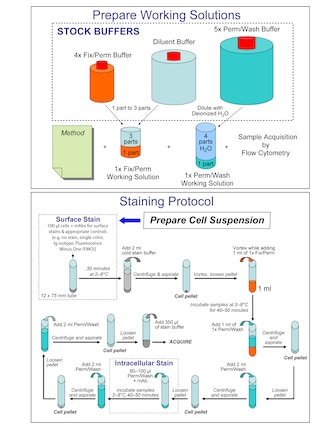
Preparation And Storage
Recommended Assay Procedures
BD® CompBeads can be used as surrogates to assess fluorescence spillover (compensation). When fluorochrome conjugated antibodies are bound to BD® CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cells and BD® CompBeads to ensure that BD® CompBeads are appropriate for your specific cellular application.
Product Notices
- When using high concentrations of antibody, background binding of this dye to erythroid fragments produced by ammonium chloride-based lysis, such as with BD Pharm Lyse™ Lysing Buffer (Cat. No. 555899), has been observed when the antibody conjugate was present during the lysis procedure. This may cause nonspecific staining of target cells, such as leukocytes, which have bound the resulting erythroid fragments. This background can be mitigated by any of the following: titrating the antibody conjugate to a lower concentration, fixing samples with formaldehyde, or removing erythrocytes before staining (eg, gradient centrifugation or pre-lysis with wash). This background has not been observed when cells were lysed with BD FACS™ Lysing Solution (Cat. No. 349202) after staining.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- This reagent has been pre-diluted for use at the recommended Volume per Test. We typically use 1 × 10^6 cells in a 100-µl experimental sample (a test).
- An isotype control should be used at the same concentration as the antibody of interest.
- Please observe the following precautions: Absorption of visible light can significantly alter the energy transfer occurring in any tandem fluorochrome conjugate; therefore, we recommend that special precautions be taken (such as wrapping vials, tubes, or racks in aluminum foil) to prevent exposure of conjugated reagents, including cells stained with those reagents, to room illumination.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
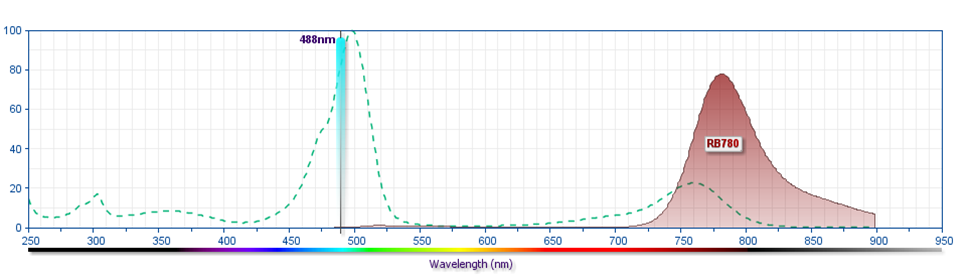
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Human donor specific background has been observed in relation to the presence of anti-polyethylene glycol (PEG) antibodies, developed as a result of certain vaccines containing PEG, including some COVID-19 vaccines. We recommend use of BD Horizon Brilliant™ Stain Buffer in your experiments to help mitigate potential background. For more information visit https://www.bdbiosciences.com/en-us/support/product-notices.
- Species cross-reactivity detected in product development may not have been confirmed on every format and/or application.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- Cy is a trademark of Global Life Sciences Solutions Germany GmbH or an affiliate doing business as Cytiva.
Companion Products


.png?imwidth=320)
The 259D/C7 monoclonal antibody specifically recognizes human Forkhead box protein P3 (FoxP3) that is also known as Scurfin. FoxP3 is encoded by FOXP3 (Forkhead box P3), likewise known as IPEX (Immune Dysregulation, Polyendocrinopathy, Enteropathy, X-Linked) and JM2, that belongs to the forkhead/winged-helix family of transcriptional regulators. FoxP3 is expressed in CD4+ regulatory T cells (Treg) and represents a specific marker for these cells. Flow cytometric analyses have shown that FoxP3 is expressed by the majority of CD4+CD25+high T cells in peripheral blood while less than half of the CD4+CD25+intermediate cell population are FoxP3 positive. Approximately 5-10% of peripheral CD4+ cells are CD4+CD25+ T regulatory cells. T regulatory cells are thought to play a critical role in the regulation of T cell-mediated immunity and to protect against autoimmunity by suppressing the proliferation and cytokine production of other T cells. In support of this hypothesis, it has been found that Foxp3 is mutated in Scurfy (sf) mice that have defective T cell tolerance leading to an X-linked lymphoproliferative disease. The 259D/C7 antibody recognizes all currently-identified isoforms of human FoxP3 and is crossreactive with FoxP3 from Cynomolgus, Rhesus and Baboon primates.
Development References (7)
-
Lauer FT, Denson JL, Beswick E, Burchiel SW. Intracellular Cytokine Detection by Flow Cytometry in Surface Marker-Defined Human Peripheral Blood Mononuclear T Cells.. Curr Protoc Toxicol. 2017; 73:18.19.1-18.19.14. (Clone-specific: Flow cytometry). View Reference
-
Law JP, Hirschkorn DF, Owen RE, Biswas HH, Norris PJ, Lanteri MC. The importance of Foxp3 antibody and fixation/permeabilization buffer combinations in identifying CD4+CD25+Foxp3+ regulatory T cells.. Cytometry A. 2009; 75(12):1040-50. (Clone-specific: Flow cytometry). View Reference
-
Mailer RKW. Alternative Splicing of FOXP3-Virtue and Vice.. Front Immunol. 2018; 9:530. (Clone-specific). View Reference
-
Roncador G, Brown PJ, Maestre L, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005; 35(6):1681-1691. (Immunogen: Flow cytometry, Immunohistochemistry, Western blot). View Reference
-
Ruitenberg JJ, Boyce C, Hingorani R, Putnam A, Ghanekar SA. Rapid assessment of in vitro expanded human regulatory T cell function.. J Immunol Methods. 2011; 372(1-2):95-106. (Clone-specific: Flow cytometry). View Reference
-
Sadlon TJ, Wilkinson BG, Pederson S, et al. Genome-wide identification of human FOXP3 target genes in natural regulatory T cells. J Immunol. 2010; 185(2):1071-1081. (Clone-specific: Flow cytometry). View Reference
-
Santegoets SJ, Dijkgraaf EM, Battaglia A, et al. Monitoring regulatory T cells in clinical samples: consensus on an essential marker set and gating strategy for regulatory T cell analysis by flow cytometry.. Cancer Immunol Immunother. 2015; 64(10):1271-86. (Clone-specific: Flow cytometry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.