Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?





Immunocytochemistry: PBMC were isolated from human peripheral blood by density gradient cetrifugation and were cultured for 2 days with plate bound anti-human CD3 and soluble anti-human CD28 in the presence of recombinant human IL-2 (Cat. No. 554603) and recombinant IL-4 (Cat. No. 554605). The cells were subsequently harvested, washed, and recultured with recombinant human IL-2 and recombinant human IL-4 for an additional 3 days. Finally, the cells were harvested, washed, and stimulated with PMA (Sigma, 5 ng/ml) and ionomycin (Sigma, 500 ng/ml) in the presence of GolgiStop™ (Cat. No. 554724) for 4 hours at 37°C. The activated cells were harvested and the level of IFN-γ producing cells were detected by a three-step staining procedure that employs a Purified Mouse Anti-Human IFN-γ antibody(Cat. No. 550011), Biotin Goat anti-mouse IgG secondary antibody (Cat. No. 550337) and Streptavidin-horseradish peroxidase (HRP)( Cat. No. 550946). (Nomarski optics, original magnification 400X).

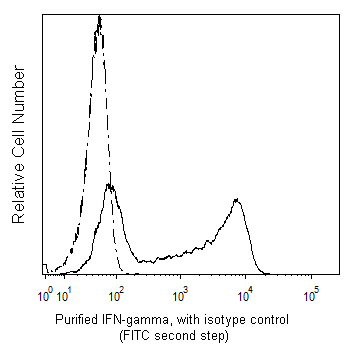
Flow cytometric analysis of IFN-γ expressed in stimulated human peripheral blood mononuclear cells. HiCK-1 Human Cytokine Positive Control Cells (Cat. No. 555061) were permeabilized with BD Perm/Wash™ Buffer (Cat. No. 554723). The cells were then stained with either Purified Mouse IgG1, κ Isotype Control (Cat No. 550878; dashed line histogram) or with Purified Mouse Anti-Human IFN-γ antibody (Cat No. 550011; solid line histogram) followed by FITC Goat Anti-Mouse IgG/IgM (Cat. No. 555988). Fluorescent histograms showing the expression of IFN-γ (or Ig Isotype Control staining) were derived from gated events with the forward and side light-scatter characteristics of intact lymphocytes. Flow cytometry was performed using a BD LSRFortessa™ system.


BD Pharmingen™ Purified Mouse Anti-Human IFN-γ

BD Pharmingen™ Purified Mouse Anti-Human IFN-γ

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
Immunocytochemistry:. For optimal indirect immunocytochemical staining, the B27 antibody should be titrated (≤ 1 µg) and visualized via a three-step staining procedure. Please see protocol below for a detailed description of the immunocytochemical procedure. The avidin/biotin method is a highly sensitive method, because it employs a mixture of avidin and biotinylated enzyme complexes to increase immunoenzymatic signals. For optimal detection of cytokine producing cells, horseradish peroxidase is the preferred enzyme system.
CYTOKINE IMMUNOCYTOCHEMISTRY PROTOCOL
REAGENTS REQUIRED
1. Fixation Buffer: 5% formalin (10% formalin, CMS, Cat. No. 245-684) is dissolved in phosphate buffered-saline (PBS) (Bacto FA Buffer, Difco Laboratories, Cat. No. 2314-15-0), or BD Pharmingen™ ICC Fixation Buffer (BD Cat. No. 550010)
2. Endogenous Peroxidase Blocking Buffer: DAKO Peroxidase Blocking Reagent (DAKO, Cat. No. S2001).
3. Endogenous Biotin Blocking Buffer: Biotin/Avidin Blocking Kit (Vector Laboratories, Cat. No. SP-2001).
4. Antibody dilution buffer: BD™ Pharmingen Antibody Diluent for IHC (Cat. No. 559148) supplemented with saponin.
5. Microscopic slides: Adhesion Slides (Erie Scientific Company, Cat. No. ER-202B-AD) or for cytospins, Colorfrost /Plus slides (Fisher, Cat. No. 12-550-17).
6. Detection system: BD Pharmingen Streptavidin-horseradish peroxidase (HRP), (Cat. No. 550946).
7. Mounting medium for short-term storage: Aqua-mount® (Lerner Laboratories, Cat. No. 13800).
8. DAB Substrate Kit (contains 3-3 -Diaminobenzidine tetra hydrochloride), (BD Cat. No. 550880)
SECONDARY ANTIBODIES
1. Biotin Goat anti-Mouse IgG (Cat. No. 550337)
PROCEDURE FOR IMMUNOCYTOCHEMICAL STAINING OF SINGLE-CELL PREPARATIONS
This procedure describes the immunoenzymatic technique of staining cytokines within individual cells that are immobilized on microscopic slides via adherence (adherent slides) or centrifugation (cytospins).
ADHESION SLIDES
1. Harvest cells and wash them twice in PBS using centrifugation (400 x g for 5 min) to remove residual protein.
2. Adjust the cell concentration at 4-5 x 10e6 cells/ml in PBS.
3. Place 20 µl of the cell suspension in each well of the adhesion slides and let them adhere at room temperature (RT) for 20 min. Please note that the slides should be washed in PBS at RT for 5 min before transferring the cells.
4. Fix cells on slides using fixation buffer for 15 min at RT.
5. Wash slides 2X in PBS with 5 min incubations.
6. Block slides with PBS supplemented with 1% (w/v) BSA (Sigma) for 30 min at RT or 10 min at 37°C.
7. Wash slides 2X in PBS and proceed with staining or air dry them and store them at -80°C for future use.
8. Incubate slides with 20 µl of 1% goat serum and PBS with 0.1% (w/v) saponin for 30 min at RT.
9. Wash slides 2X with PBS with 5 min incubations.
10. Block endogenous peroxidase activity with Endogenous Peroxidase Blocking Buffer (20 µl/well) for 10 min at RT.
11. Wash 2X in PBS with 5 min incubations.
12. Incubate each well with Avidin (20 µl/well) for 15 min.
13. Wash 2X in PBS with 5 min incubations.
14. Incubate each well with Biotin (20 µl/well) for 15 min.
15. Wash 2X in PBS with 5 min incubations.
16. Incubate each well for 1 hr at RT with 20 µl of purified cytokine-specific antibody or appropriate immunoglobulin isotype control diluted in Pharmingen's IHC Diluent Buffer supplemented with saponin.
17. Wash slides 2X in PBS with 5 min incubations.
18. Incubate each well with 20 µl of a biotinylated secondary antibody diluted in IHC Cytokine Diluent Buffer for 30 min at RT.
19. Wash 2X in PBS with 5 min incubations.
20. Apply 20 µl of Streptavidin-HRP (BD Cat. No. 550946) to each well on slides and incubate for 30 min at RT.
21. Wash slides 2X with PBS with 5 minutes incubations.
22. Incubate with DAB Substrate as directed, (BD Cat. No. 550880) for less than 5 min at RT.
23. Stop the development of the color reaction by washing with PBS.
24. The slides are subsequently mounted in short-term storage mounding medium.
CYTOSPINS
1. Assemble the Cytospin's sample chamber (e.g. Cytospin 3, Shandon, UK or comparable centrifuge), filter card, slide and cytospin racks according to manufacturer's specifications.
2. Load 40 µl of approximately 1 x 10e6 cells to each sample chamber.
3. Spin slides at 600 rpm for 2 min.
4. Take slides out of the cytospin rack and place them on a staining rack.
5. For fixation and staining please follow the steps 4 through 24 specified above for staining cells on adhesion slides.
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Sodium azide is a reversible inhibitor of oxidative metabolism; therefore, antibody preparations containing this preservative agent must not be used in cell cultures nor injected into animals. Sodium azide may be removed by washing stained cells or plate-bound antibody or dialyzing soluble antibody in sodium azide-free buffer. Since endotoxin may also affect the results of functional studies, we recommend the NA/LE (No Azide/Low Endotoxin) antibody format, if available, for in vitro and in vivo use.
- Species cross-reactivity detected in product development may not have been confirmed on every format and/or application.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products




The B27 monoclonal antibody specifically binds to human interferon-γ (IFN-γ), a 14-18 kDa glycoprotein containing 143 amino acid residues. IFN-γ is a potent multifunctional cytokine produced by several activated cell types including NK, NKT, CD4+TCRαβ+, CD8+TCRαβ+, and TCRγδ+ T cells. IFN-γ exerts its biological effects through specific binding to the high-affinity IFN-γ receptor complex comprised of IFN-γRα (CD119) and IFN-γRβ subunits. In addition to its antiviral effects, IFN-γ upregulates a number of lymphoid cell functions including the antimicrobial and anti-tumor responses of macrophages, NK cells, and neutrophils. In addition, IFN-γ influences the regulation of proliferation, differentiation, and effector responses of B cell and T cell subsets. These influences can involve IFN-γ's capacity to boost MHC class I and II expression by antigen-presenting cells as well as direct effects on B cells and T cells themselves. B27 is a neutralizing antibody. The use of B27 antibody for epitope mapping of human IFN-γ has been described. The B27 antibody has been reported not to bind to denatured IFN-γ.
Development References (6)
-
Abrams JS, Roncarolo MG, Yssel H, Andersson U, Gleich GJ, Silver JE. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992; 127:5-24. (Clone-specific). View Reference
-
Favre C, Wijdenes J, Cabrillat H, Djossou O, Banchereau J, de Vries JE. Epitope mapping of recombinant human gamma interferon using monoclonal antibodies. Mol Immunol. 1989; 26(1):17-25. (Clone-specific: Flow cytometry, Immunoprecipitation, Neutralization). View Reference
-
Fonteneau JF, Le Drean E, Le Guiner S, Gervois N, Diez E, Jotereau F. Heterogeneity of biologic responses of melanoma-specific CTL. J Immunol. 1997; 159(6):2831-2839. (Biology). View Reference
-
Hsu SM, Raine L, Fanger H. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am J Clin Pathol. 1981; 75(5):734-738. (Methodology: Immunocytochemistry (cytospins)). View Reference
-
Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981; 29(4):577-580. (Methodology: Immunocytochemistry (cytospins)). View Reference
-
Rotteveel FT, Kokkelink I, van Lier RA, et al. Clonal analysis of functionally distinct human CD4+ T cell subsets. J Exp Med. 1988; 168(5):1659-1673. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.