Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


.png)

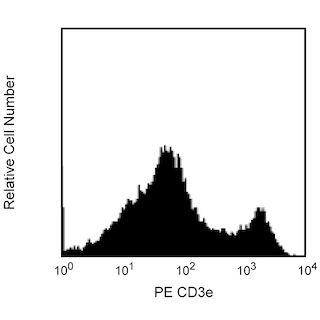
Two-color analysis of the expression of Vβ 14 TCR on peripheral T lymphocytes. C57BL/6 lymph node cells were incubated simultaneously with biotinylated 14-2 and PE-conjugated 145-2C11 (anti-CD3e, Cat. No. 553063/553064) monoclonal antibodies, followed by Avidin-FITC (Cat. No. 554057). Flow cytometry was performed on a FACScan™ (BDIS, San Jose, CA).
.png)

BD Pharmingen™ FITC Rat Anti-Mouse Vβ 14 T-Cell Receptor
.png)
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
For flow cytometry of cell suspensions from peripheral lymphoid tissue, it is recommended that multicolor staining be performed to distinguish T lymphocytes from non-T cells.
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
Companion Products
.png?imwidth=320)
The 14-2 antibody reacts with the Vβ 14 T-Cell Receptor (TCR) of mice having the a (e.g., C57BR, C57L, SJL, SWR), b (e.g., A, AKR, BALB/c, CBA, C3H/He, C57BL, C58, DBA/1, DBA/2), and c (e.g., RIII) halpotypes of the Tcrb gene complex. Vβ 14 TCR-expressing T lymphocytes are completely eliminated in mice expressing I-E and the superantigens encoded by Mtv-2 endogenous provirus and/or MMTV-C3H, MMTV-GR, or MMTV-D2.GD exogenous virus. Recognition of these determinants by Vβ 14 TCR-expressing T cells is dependent upon presentation by I-E. Plate bound 14-2 antibody activates Vβ 14 TCR-bearing T cells.
This antibody is routinely tested by flow cytometric analysis. Other applications were tested at BD Biosciences Pharmingen during antibody development only or reported in the literature.
Development References (7)
-
Acha-Orbea H, Shakhov AN, Scarpellino L, et al. Clonal deletion of V beta 14-bearing T cells in mice transgenic for mammary tumour virus. Nature. 1991; 350(6315):207-211. (Biology). View Reference
-
Choi Y, Kappler JW, Marrack P. A superantigen encoded in the open reading frame of the 3' long terminal repeat of mouse mammary tumour virus. Nature. 1991; 350(6315):203-207. (Biology). View Reference
-
Golovkina TV, Chervonsky A, Dudley JP, Ross SR. Transgenic mouse mammary tumor virus superantigen expression prevents viral infection. Cell. 1992; 69(4):637-645. (Clone-specific). View Reference
-
Hodes RJ, Abe R. Mouse endogenous superantigens: Ms and Mls-like determinants encoded by mouse retroviruses.. Curr Protoc Immunol. 2001; Appendix 1:Appendix 1F. (Biology). View Reference
-
Liao NS, Maltzman J, Raulet DH. Positive selection determines T cell receptor V beta 14 gene usage by CD8+ T cells. J Exp Med. 1989; 170(1):135-143. (Immunogen). View Reference
-
Marrack P, Kushnir E, Kappler J. A maternally inherited superantigen encoded by a mammary tumour virus. Nature. 1991; 349(6309):524-526. (Biology). View Reference
-
Tomonari K, Fairchild S, Rosenwasser OA. Influence of viral superantigens on V beta- and V alpha-specific positive and negative selection. Immunol Rev. 1993; 131:131-168. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.