Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


Anti-HLA-DR PerCP-Cy™5.5
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
The antibody reagent is stable until the expiration date shown on the label when stored at 2°C to 8°C. Do not use after the expiration date. Do not freeze the reagent or expose it to direct light during storage or incubation with cells. Keep the outside of the reagent vial dry.
Do not use the reagent if you observe any change in appearance. Precipitation or discoloration indicates instability or deterioration.
Anti–HLA-DR is intended for in vitro diagnostic use in the identification of cells expressing the HLA-DR antigen, using a BD FACS™ brand flow cytometer. The flow cytometer must be equipped to detect light scatter and the appropriate fluorescence, and be equipped with appropriate software for data acquisition and analysis. See your instrument user’s guide for instructions.
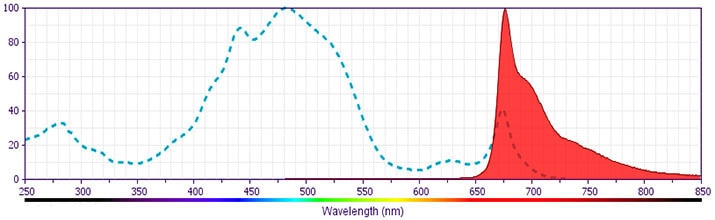
Development References (31)
-
Borowitz MJ, Gockerman JP, Moore JO, et al. Clinicopathologic and cytogenic features of CD34 (My 10)-positive acute nonlymphocytic leukemia. Am J Clin Pathol. 1989; 91:265-270. (Biology).
-
Brodsky FM. A matrix approach to human class II histocompatibility antigens: reactions of four monoclonal antibodies with the products of nine haplotypes.. Immunogenetics. 1984; 19(3):179-94. (Biology). View Reference
-
Centers for Disease Control. Update: universal precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other bloodborne pathogens in healthcare settings. MMWR. 1988; 37:377-388. (Biology).
-
Clinical Applications of Flow Cytometry: Quality Assurance and Immunophenotyping of Lymphocytes: Approved Guideline. H42-A2. 2007. (Biology).
-
Clinical and Laboratory Standards Institute. 2005. (Biology).
-
Consensus protocol for the flow cytometric immunophenotyping of hematopoietic malignancies. Rothe G, Schmitz G. Leukemia. 1996; 10:877-895. (Biology).
-
Dorak MT, Lawson T, Machulla HK, Darke C, Mills KI, Burnett AK. Unravelling an HLA-DR association in childhood acute lymphoblastic leukemia. Blood. 1999; 94:694-700. (Biology).
-
Edwards JA, Durant BM, Jones DB, Evans PR, Smith JL. Differential expression of HLA class II antigens in fetal human spleen: relationship of HLA-DP, DQ, and DR to immunoglobulin expression.. J Immunol. 1986; 137(2):490-7. (Biology). View Reference
-
Engleman EG, Warnke R, Fox RI, Dilley J, Benike CJ, Levy R. Studies of a human T lymphocyte antigen recognized by a monoclonal antibody.. Proc Natl Acad Sci USA. 1981; 78(3):1791-5. (Biology). View Reference
-
Geisberg M, Stole E, Knowles R. Knapp W, Dörken B, Gilks WR, et al, ed. Leucocyte Typing IV: White Cell Differentiation Antigens. New York, NY: Oxford University Press Inc; 1989:729-736.
-
Hulstaert F, Hannet I, Deneys V, et al. Age-related changes in human blood lymphocyte subpopulations, II: Varying kinetics of percentage and absolute count measurements. Clin Immunol Immunopathol. 1994; 70:152-158. (Biology).
-
Jackson AL, Warner NL. Rose NR, Friedman H, Fahey JL, ed. Manual of Clincial Laboratory Immunology, Third Edition. Washington DC: American Society for Microbiology; 1986:226-235.
-
Lampson LA, Levy R. Two populations of Ia-like molecules on a human B cell line.. J Immunol. 1980; 125(1):293-9. (Biology). View Reference
-
Lazarchick J, Hopkins M. HLA-DR negative acute non-lymphocytic leukemia. Ann Clin Lab Sci. 1998; 28:150-152. (Biology).
-
Levacher M, Tallet S, Dazza MC, Dournon E, Rouveix B, Pocidalo JJ. T activation marker evaluation in ARC patients treated with AZT. Comparison with CD4+ lymphocyte count in non-progressors and progressors towards AIDS.. Clin Exp Immunol. 1990; 81(2):177-82. (Biology). View Reference
-
Macedo A, Orfao A, Gonzalez M, et al. Immunological detection of blast cell subpopulations in acute myeloblastic leukemia at diagnosis: implications for minimal residual disease studies. Leukemia. 1995; 9:993-998. (Biology).
-
O'Gormon MRG, Millard DD, Lowder JN, Yogev R. Lymphocyte subpopulations in healthy 1–3-day-old infants. Cytometry. 1998; 34:235-241. (Biology).
-
Paietta E, Andersen J, Gallagher R, et al. The immunophenotype of acute promyelocytic leukemia (APL): an ECOG study. Leukemia. 1994; 8:1108-1112. (Biology).
-
Porwit-MacDonald A, Janossy G, Ivory K, et al. Leukemia-associated changes identified by quantitative flow cytometry, IV: CD34 overexpression in acute myelogenous leukemia M2 with t(8;21). Blood. 1996; 87:1162-1169. (Biology).
-
Schuerch CI, Fleetwood M, Glidewell O, Goodspeed N, Maier B. Lymphocyte subsets and activation antigens in a reference population: a flow cytometric study using single and double antibody staining. Immunol Invest. 1987; 16:345-360. (Biology).
-
Shahabuddin S. Quantitative differences in CD8+ lymphocytes, CD4/CD8 ratio, NK cells, and HLADR+- activated T cells of racially different male populations. Clin Immunol Immunopathol. 1995; 75:168-170. (Biology).
-
Stelzer GT, Marti G, Hurley A, McCoy PJ, Lovett EJ, Schwartz A. US-Canadian consensus recommendations on the immunophenotypic analysis of hematologic neoplasia by flow cytometry: standardization and validation of laboratory procedures. Cytometry. 1997; 30:214-230. (Biology).
-
Stites DP, Casavant CH, McHugh TM, et al. Flow cytometric analysis of lymphocyte phenotypes in AIDS using monoclonal antibodies and simultaneous dual immunofluorescence.. Clin Immunol Immunopathol. 1986; 38(2):161-77. (Biology). View Reference
-
Terstappen LW, Hollander Z, Meiners H, Loken MR. Quantitative comparison of myeloid antigens on five lineages of mature peripheral blood cells. J Leukoc Biol. 1990; 48(2):138-148. (Biology). View Reference
-
Tollerud DJ, Ildstad ST, Morris Brown L, et al. T-cell subsets in healthy teenagers: transition to the adult phenotype. Clin Immunol Immunopathol. 1990; 56:88-96. (Biology).
-
Tomkinson BE, Wagner DK, Nelson DL, Sullivan JL. Activated lymphocytes during acute Epstein-Barr virus infection.. J Immunol. 1987; 139(11):3802-7. (Biology). View Reference
-
Vaughan WP, Civin CI, Weisenburger DD, et al. Acute leukemia expressing the normal human hematopoietic stem cell membrane glycoprotein CD34 (MY10). Leukemia. 1988; 2:661-666. (Biology).
-
Venditti A, Del Poeta G, Stasi R, et al. Triple immunofluorescence evaluation of CD15, CD34 and class II expression by flow cytometry in normal and leukemic bone marrows. Haematologica. 1993; 78:359-363. (Biology).
-
Warnke R, Miller R, Grogan T, Pederson M, Dilley J, Levy R. Immunologic phenotype in 30 patients with diffuse large-cell lymphoma.. N Engl J Med. 1980; 303(6):293-300. (Biology). View Reference
-
Warnke RA, Levy R. Detection of T and B cell antigens with hybridoma monoclonal antibodies: a biotinavidin-horseradish peroxidase method. J Histochem Cytochem. 1980; 28:771-776. (Biology).
-
Zipf TF, Fox RI, Dilley J, Levy R. Definition of the high-risk acute lymphoblastic leukemia patient by immunological phenotyping with monoclonal antibodies.. Cancer Res. 1981; 41(11 Pt 2):4786-9. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For In Vitro Diagnostic Use.
23-22942-00
![]()
Documents are subject to revision without notice. Please verify you have the correct revision of the document, and always refer back to BD's eIFU website for the latest and most up to date information.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.