-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- Spain (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
BD Phosflow™ Perm Buffer IV 10×

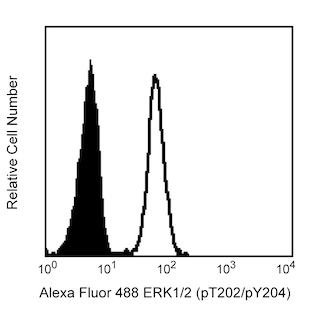
Immunofluorescent staining of human and mouse lymphocytes after permeabilization with Perm Buffer IV. Human whole blood (top row) and mouse splenocytes (bottom row) were treated with 100 ng/ml IL-2 (Cat. No. 554603, top right figure) or 100 ng/ml IL-6 (Cat. No. 550071, bottom right figure) or left untreated (all 4 figures) for 15 minutes. Erythrocytes were lysed and leukocytes were fixed and permeabilized with Perm Buffer IV at either 1× or 0.5× according to the Recommended Assay Procedure. Then the human cells were stained with PE Mouse Anti-Human CD19 (Cat. No. 555413, top panel of top left figure), PE Mouse Anti-Human CD4 (Cat. No. 347327, bottom panel of top left figure), or PE Mouse Anti-Stat5 (pY694) (Cat. No. 612567, top right figure). Similarly, the mouse cells were stained with APC Hamster Anti-Mouse CD3e (Cat. No. 553066) and Alexa Fluor® 488 Rat Anti-Mouse CD45R (Cat. No. 557669) in the presence of Purified Rat Anti-Mouse CD16/CD32 (Mouse BD Fc Block™, Cat. No. 553142, bottom left figure) or PE Mouse Anti-Stat3 (pY705) (Cat. No. 612569, bottom right figure). Additional controls for the cell-surface staining were live cells that had undergone erythrocyte lysis using BD Pharm Lyse™ lysing buffer (Cat. No. 555899, left column) but no fixation or permeabilization. The fold change is indicated for staining of Stat5 (pY694) or Stat3 (pY705) with each perm buffer concentration (upper right corners of panels in the right column). Lymphocytes were selected by scatter profile. Flow cytometry was performed on a BD FACSCanto™ II flow cytometer.

Immunofluorescent staining of human and mouse lymphocytes after permeabilization with Perm Buffer IV. Human whole blood (top row) and mouse splenocytes (bottom row) were treated with 100 ng/ml IL-2 (Cat. No. 554603, top right figure) or 100 ng/ml IL-6 (Cat. No. 550071, bottom right figure) or left untreated (all 4 figures) for 15 minutes. Erythrocytes were lysed and leukocytes were fixed and permeabilized with Perm Buffer IV at either 1× or 0.5× according to the Recommended Assay Procedure. Then the human cells were stained with PE Mouse Anti-Human CD19 (Cat. No. 555413, top panel of top left figure), PE Mouse Anti-Human CD4 (Cat. No. 347327, bottom panel of top left figure), or PE Mouse Anti-Stat5 (pY694) (Cat. No. 612567, top right figure). Similarly, the mouse cells were stained with APC Hamster Anti-Mouse CD3e (Cat. No. 553066) and Alexa Fluor® 488 Rat Anti-Mouse CD45R (Cat. No. 557669) in the presence of Purified Rat Anti-Mouse CD16/CD32 (Mouse BD Fc Block™, Cat. No. 553142, bottom left figure) or PE Mouse Anti-Stat3 (pY705) (Cat. No. 612569, bottom right figure). Additional controls for the cell-surface staining were live cells that had undergone erythrocyte lysis using BD Pharm Lyse™ lysing buffer (Cat. No. 555899, left column) but no fixation or permeabilization. The fold change is indicated for staining of Stat5 (pY694) or Stat3 (pY705) with each perm buffer concentration (upper right corners of panels in the right column). Lymphocytes were selected by scatter profile. Flow cytometry was performed on a BD FACSCanto™ II flow cytometer.

Immunofluorescent staining of human and mouse lymphocytes after permeabilization with Perm Buffer IV. Human whole blood (top row) and mouse splenocytes (bottom row) were treated with 100 ng/ml IL-2 (Cat. No. 554603, top right figure) or 100 ng/ml IL-6 (Cat. No. 550071, bottom right figure) or left untreated (all 4 figures) for 15 minutes. Erythrocytes were lysed and leukocytes were fixed and permeabilized with Perm Buffer IV at either 1× or 0.5× according to the Recommended Assay Procedure. Then the human cells were stained with PE Mouse Anti-Human CD19 (Cat. No. 555413, top panel of top left figure), PE Mouse Anti-Human CD4 (Cat. No. 347327, bottom panel of top left figure), or PE Mouse Anti-Stat5 (pY694) (Cat. No. 612567, top right figure). Similarly, the mouse cells were stained with APC Hamster Anti-Mouse CD3e (Cat. No. 553066) and Alexa Fluor® 488 Rat Anti-Mouse CD45R (Cat. No. 557669) in the presence of Purified Rat Anti-Mouse CD16/CD32 (Mouse BD Fc Block™, Cat. No. 553142, bottom left figure) or PE Mouse Anti-Stat3 (pY705) (Cat. No. 612569, bottom right figure). Additional controls for the cell-surface staining were live cells that had undergone erythrocyte lysis using BD Pharm Lyse™ lysing buffer (Cat. No. 555899, left column) but no fixation or permeabilization. The fold change is indicated for staining of Stat5 (pY694) or Stat3 (pY705) with each perm buffer concentration (upper right corners of panels in the right column). Lymphocytes were selected by scatter profile. Flow cytometry was performed on a BD FACSCanto™ II flow cytometer.

BD™ Phosflow Perm Buffer IV 10×
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Product Details
Description
BD Phosflow™ Perm Buffer IV (Perm Buffer IV) is intended for the permeabilization of paraformaldehyde-fixed single-cell suspensions prepared from lymphoid tissues, peripheral blood mononuclear cells (PBMC) and lysed whole blood cells. Permeabilization of cells by treatment with this buffer and subsequent washing with staining buffer enables access for fluorescent antibodies to stain intracellular molecules. This buffer preserves the light scattering properties of cells while providing the capacity for intracellular immunofluorescent staining in preparation for Phosflow cytometric analysis.
Perm Buffer IV is formulated for improved immunofluorescent staining and detection of certain intracellular phosphorylated proteins. PBMC, for example, can be treated with protein kinase activators (eg, phorbol esters) or inhibitors, mitogens or cytokines and fixed with BD
Cytofix™ Fixation Buffer (Cat. No. 554655). In the case of peripheral whole blood cells, the blood cells can be treated, erythrocytes lysed and the remaining leukocytes fixed using BD Phosflow™ Lyse/Fix Buffer (Cat. No. 558049). Following fixation, the cells can then be permeabilized with Perm Buffer IV and washed with staining buffer to allow for effective intracellular staining. Designed for optimal staining of cells destined for flow cytometric analysis, Perm Buffer IV can be used with fluorescent antibodies specific for phosphorylated cell signaling molecules to define the nature of signaling pathways induced by individual cells responding to various activators, inhibitors, or combinations thereof. At a 1.0× concentration, Perm Buffer IV has been shown to support large fold changes (i.e., mean fluorescent intensity staining ratios of treated versus untreated cell populations) for phosphorylated Signal transducer and activators of transcription (Stat) proteins. However, it should be noted that at a 1.0× concentration, Perm Buffer IV may result in a greater cell loss (~18% to 48%) than other BD permeabilization buffers and decreased ability to stain certain cell-surface markers. At a 0.5× concentration, the BD Phosflow™ Perm Buffer IV has been shown to be compatible with the immunofluorescent staining of phosphorylated intracellular Stat proteins, although sometimes with lower fold changes. Unlike the 1.0× concentration of Perm Buffer IV, the 0.5× concentration of Buffer IV offers improved compatibility for staining cell-surface CD markers and results in comparable cell recoveries when compared with the BD Phosflow™ Perm Buffers I, II, and III. The figures demonstrate some examples of intracellular and cell-surface staining of cells that were permeabilized with Perm Buffer IV at 1× and 0.5× concentrations.
Preparation And Storage
Recommended Assay Procedures
This is the standard protocol for permeabilizing fixed leukocytes with Perm Buffer IV for subsequent staining of cell-surface markers and phosphorylated signaling proteins.
1. Prewarm BD Cytofix™ Fixation Buffer (Cat. No. 554655), for PBMC, or BD Phosflow™ Lyse/Fix Buffer (Cat. No. 558049), for whole
blood or splenocytes, to 37°C prior to use.
2. Dilute Perm Buffer IV to 1.0× using 1× Phosphate Buffered Saline (PBS) prior to use.
3. Prepare cells. Use step a for PBMC or step b for whole blood or splenocytes.
a. Isolate PBMC by density gradient separation (eg, using BD Vacutainer® CPT Cell Preparation Tube with Sodium Heparin, Cat. No. 362753) of whole blood, wash the cells well, and then treat them with the desired activator, inhibitor or combinations thereof. At the end of the cell treatment, immediately mix one volume of the warmed BD Cytofix Fixation Buffer with one volume of the suspended PBMC. Mix well and incubate the tubes in a 37°C water bath for 10 to 15 minutes. Spin down the cells at 400g for 10 minutes in a table-top centrifuge. Aspirate the supernatant.
or
b. Obtain whole blood or suspend spleen cells. Treat the cells (eg, 100 µL) with the desired activator and/or inhibitor. After treatment, add a 20-fold excess volume (eg, 2.0 ml) of warmed BD Phosflow™ Lyse/Fix Buffer. Mix well and incubate the tubes in a 37°C water bath for 10 to 15 minutes. Spin down the cells at 400g for 10 minutes in a table-top centrifuge. Aspirate the supernatant.
4. Wash the fixed leukocytes once with PBS. Pellet the cells by centrifugation at 400g and remove all the supernatant.
Note: High residual volumes of supernatant left over after the centrifugation and aspiration steps may dilute the Perm Buffer IV that is subsequently added. This may result in poor staining profiles.
5. Vortex the tubes to loosen the cells. Permeabilize the cells by slowly adding Perm Buffer IV dropwise to the cells. Add approximately 10 ml of Perm Buffer IV per 5 to 10 million cells. Add a minimum of 1ml of Perm Buffer IV for cell concentration less than 5 million cells. Incubate at room temperature for 15 to 20 minutes.
Note 1: Longer permeabilization time or using a ratio of cell to buffer volume outside the recommended ratio may result in poorer fluorescent surface marker- and/or phosphorylated protein-specific antibody staining and detection.
Note 2: Permeabilization with Perm Buffer IV may result in decreased cell recovery.
6. Centrifuge at 400g for 10 minutes and remove the supernatant by aspiration.
7. Wash twice by adding BD Pharmingen™ Stain Buffer (FBS) to the cells. Centrifuge at 400g for 10 minutes and remove the supernatant by aspiration each time.
8. Resuspend the cells in BD Pharmingen™ Stain Buffer (FBS) at 10X10^6 cells/ml and aliquot 100 µl to each sample tube to continue with antibody staining and flow cytometric analysis.
It should also be noted that if staining of cell surface markers after cellular permeabilization with Perm Buffer IV does not work well, then the cells can be pre-stained with fluorescent antibodies directed against surface markers prior to cellular fixation or between the fixation and permeabilization steps. Staining can then be performed with antibodies specific for phosphorylated intracellular signaling molecules.
Product Notices
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Alexa Fluor® is a registered trademark of Molecular Probes, Inc., Eugene, OR.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
Data Sheets
Companion Products


Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.