-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- Spain (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
BD Phosflow™ Lyse/Fix Buffer 5X

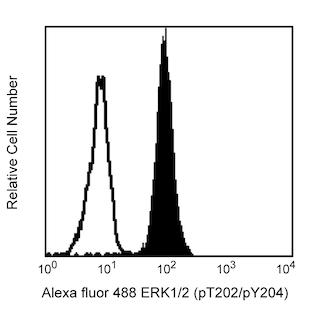
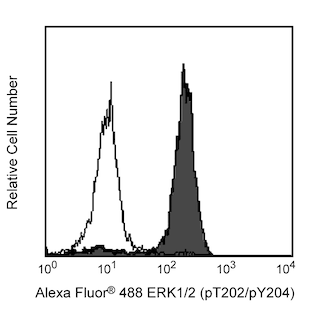
Flow cytometric analysis of ERK1/2 (pT202/pY204) on human whole blood treated with BD Phosflow™ Lyse/Fix Buffer. Freshly isolated human whole blood was left either untreated (shaded histogram) or treated with 400 nM PMA at 37°C for 10 minutes (open histogram), then fixed with Lyse/Fix Buffer 5X (Cat. No. 558049). Leukocytes were spun down and washed once with Hank's solution, followed by permeabilization with BD Phosflow™ Perm Buffer II (Cat. No. 558052) for 30 minutes. After washing the cells twice with BD Pharmingen™ Stain Buffer (Cat. No. 554656554657), the cells were stained with Alexa Fluor® 488 mouse anti-ERK1/2 (pT202/pY204) (Cat. No. 612592). Fluorescence histograms depicting ERK1/2 (pT202/pY204) expression were derived from gated events with the forward and side light-scatter characteristics of viable lymphocytes.


Flow cytometric analysis of ERK1/2 (pT202/pY204) on human whole blood treated with BD Phosflow™ Lyse/Fix Buffer. Freshly isolated human whole blood was left either untreated (shaded histogram) or treated with 400 nM PMA at 37°C for 10 minutes (open histogram), then fixed with Lyse/Fix Buffer 5X (Cat. No. 558049). Leukocytes were spun down and washed once with Hank's solution, followed by permeabilization with BD Phosflow™ Perm Buffer II (Cat. No. 558052) for 30 minutes. After washing the cells twice with BD Pharmingen™ Stain Buffer (Cat. No. 554656554657), the cells were stained with Alexa Fluor® 488 mouse anti-ERK1/2 (pT202/pY204) (Cat. No. 612592). Fluorescence histograms depicting ERK1/2 (pT202/pY204) expression were derived from gated events with the forward and side light-scatter characteristics of viable lymphocytes.

Flow cytometric analysis of ERK1/2 (pT202/pY204) on human whole blood treated with BD Phosflow™ Lyse/Fix Buffer. Freshly isolated human whole blood was left either untreated (shaded histogram) or treated with 400 nM PMA at 37°C for 10 minutes (open histogram), then fixed with Lyse/Fix Buffer 5X (Cat. No. 558049). Leukocytes were spun down and washed once with Hank's solution, followed by permeabilization with BD Phosflow™ Perm Buffer II (Cat. No. 558052) for 30 minutes. After washing the cells twice with BD Pharmingen™ Stain Buffer (Cat. No. 554656554657), the cells were stained with Alexa Fluor® 488 mouse anti-ERK1/2 (pT202/pY204) (Cat. No. 612592). Fluorescence histograms depicting ERK1/2 (pT202/pY204) expression were derived from gated events with the forward and side light-scatter characteristics of viable lymphocytes.


BD™ Phosflow Lyse/Fix Buffer 5X

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Product Details
Description
BD Phosflow™ Lyse/Fix Buffer is intended to be used for the lysing and fixing of whole blood for use with intracellular flow cytometry. For example, after cell stimulation with kinase activators, such as phorbol esters, the BD Phosflow™ Lyse/Fix buffer can be used for lysing red blood cells and for fixing leukocytes in one step. This allows investigators to rapidly inactivate kinases, phosphatases and/or proteases, so that the in vivo phosphorylation state of the cell can be examined. The BD Phosflow™ Lyse/Fix Buffer has been reported to preserve the light scattering properties of the cell and may be used on human and mouse whole blood. Whole blood lysis has been shown to be as effective as density gradient centrifugation in the preparation of PBMCs for lymphocyte subset analysis. In many cases, phospho-specific antibodies may be used simultaneously with the staining of cell surface markers (e.g CD markers). Not all cell surface markers, however, are compatible with the BD Phosflow™ Lyse/Fix Buffer. For a current listing of compatible cell surface markers for use with BD Phosflow™ Lyse/Fix Buffer, investigators are encouraged to visit the following website: http://www.bdbiosciences.com/documents/antibodies_human_cellsurface_marker.pdf.
Preparation And Storage
Recommended Assay Procedures
Flow cytometry:
1. Collect normal human blood in the presence of EDTA or heparin and treat with appropriate activators where applicable.
2. Dilute the required amount of BD Phosflow™ Lyse/Fix Buffer (5X concentrate) 1:5 with deionoized or distilled water (at room temperature) and then pre-warm the solution to 37°C. The 1X working solution should be made fresh for each experiment and any remaining solution at the end of the experiment should be discarded.
3. Mix one volume of blood with 20 volumes of the pre-warmed BD Phosflow™ Lyse/Fix Buffer (1X). Mix well by vigorously inverting the tubes 8-10 times, and then incubate the tubes in a 37°C water bath for 10 min.
4. Spin down the cells at 500x g for 8 min in a table-top centrifuge, aspirate the supernatant, and wash the cells once with Hank's solution.
5. Vortex to loosen the cells and permeablize the cells by adding the appropriate permeabilization reagent, such as cold BD Phosflow™ Perm Buffer II, slowly while vortexing. Incubate on ice for 30 min and then spin down the cells.
6. Wash the cells twice and resuspend with BD Pharmingen™ Staining Buffer.
7. Stain the cells with appropriate antibodies for 30-60 min at room temperature, wash and prepare for flow cytometric analysis.
Danger: BD Phosflow™ Lyse/Fix Buffer contains 7.15% methanol (w/w), 20.35% formaldehyde (w/w) and 15.65% diethylene glycol (w/w).
Hazard Statements:
Combustible liquid.
Harmful if swallowed or in contact with skin.
Toxic if inhaled.
Causes skin irritation.
Causes serious eye damage.
May cause an allergic skin reaction.
Suspected of causing genetic defects.
May cause cancer. Route of exposure: Inhalative.
May cause damage to organs. May cause respiratory irritation.
May cause damage to the kidneys through prolonged or repeated exposure. Route of exposure: Oral.
Precautionary Statements:
Obtain special instructions before use.
Wear protective gloves / eye protection.Wear protective clothing.
Do not breathe mist/vapours/spray.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do.
Continue rinsing.
IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing. Get immediate medical advice/attention.
IF exposed or concerned: Get medical advice/attention.
Store in a well-ventilated place. Keep container tightly closed.
Product Notices
- The Alexa Fluor®, Pacific Blue™, and Cascade Blue® dye antibody conjugates in this product are sold under license from Molecular Probes, Inc. for research use only, excluding use in combination with microarrays, or as analyte specific reagents. The Alexa Fluor® dyes (except for Alexa Fluor® 430), Pacific Blue™ dye, and Cascade Blue® dye are covered by pending and issued patents.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Data Sheets
Companion Products


Development References (3)
-
Ashmore LM, Shopp GM, Edwards BS. Lymphocyte subset analysis by flow cytometry. Comparison of three different staining techniques and effects of blood storage. J Immunol Methods. 1989; 118(2):209-215. (Biology). View Reference
-
De Paoli P, Reitano M, Battistin S, Castiglia C, Santini G. Enumeration of human lymphocyte subsets by monoclonal antibodies and flow cytometry: a comparative study using whole blood or mononuclear cells separated by density gradient centrifugation. J Immunol Methods. 1984; 72(2):349-353. (Biology). View Reference
-
Renzi P, Ginns LC. Analysis of T cell subsets in normal adults. Comparison of whole blood lysis technique to Ficoll-Hypaque separation by flow cytometry. J Immunol Methods. 1987; 98(1):53-56. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.