-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- Germany (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
BD Horizon™ Brilliant Stain Buffer Plus
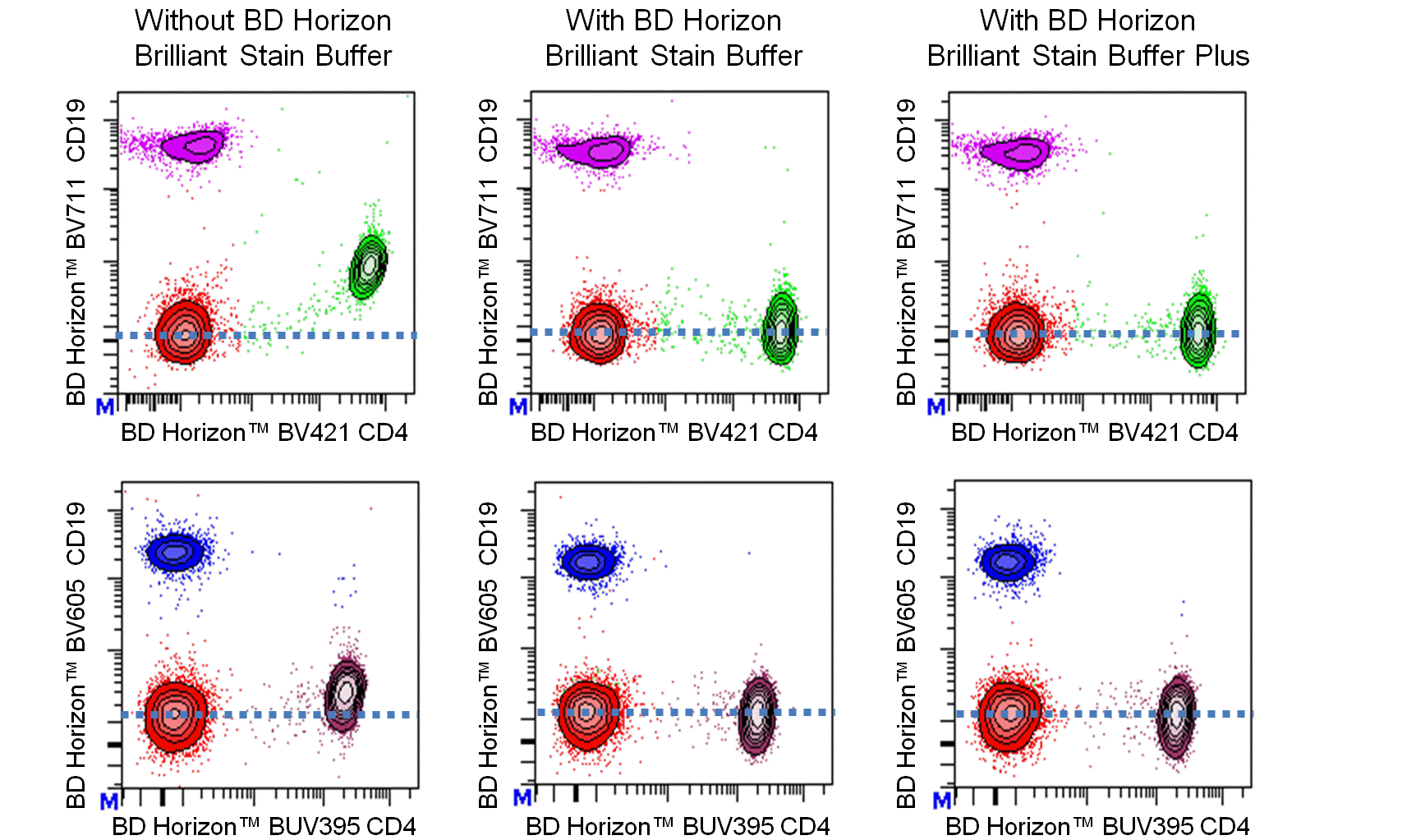
Figure 1. Either BD Horizon Brilliant Stain Buffer or BD Horizon Brilliant Stain Buffer Plus should be used in multicolor immunofluorescent staining and flow cytometric analysis when using two or more BD Horizon Brilliant reagents. Whole human blood was stained with BD Horizon™ BV421 Mouse Anti-Human CD4 and BD Horizon™ BV711 Mouse Anti-Human CD19 (Top Plots) antibodies or BD Horizon™ BUV395 Mouse Anti-Human CD4 and BD Horizon™ BV605 Mouse Anti-Human CD19 (Bottom Plots) antibodies. Erythrocytes were lysed with BD FACS Lysing Solution (Cat. No. 349202). Two-color flow cytometric contour plots showing the correlated expression of CD4 versus CD19 were derived from gated events with the forward and side light-scatter characteristics of intact lymphocytes. Staining cells in the presence of BD Horizon Brilliant Stain Buffer (Cat. No. 563794/566349; Middle Plots) or BD Horizon Brilliant Stain Buffer Plus (Cat. No. 563795; Right Plots) restores the major lymphocyte populations to their expected fluorescent staining patterns (dotted lines) when compared with cells stained without a Brilliant Stain Buffer (Left Plots). Flow cytometric analysis was performed using a BD LSRFortessa™ Cell Analyzer System.

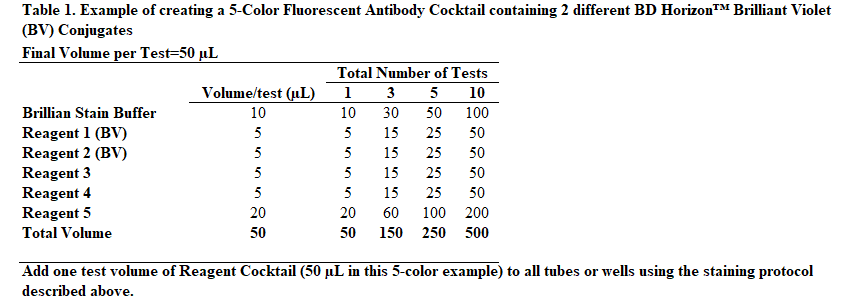
Figure 1. Either BD Horizon Brilliant Stain Buffer or BD Horizon Brilliant Stain Buffer Plus should be used in multicolor immunofluorescent staining and flow cytometric analysis when using two or more BD Horizon Brilliant reagents. Whole human blood was stained with BD Horizon™ BV421 Mouse Anti-Human CD4 and BD Horizon™ BV711 Mouse Anti-Human CD19 (Top Plots) antibodies or BD Horizon™ BUV395 Mouse Anti-Human CD4 and BD Horizon™ BV605 Mouse Anti-Human CD19 (Bottom Plots) antibodies. Erythrocytes were lysed with BD FACS Lysing Solution (Cat. No. 349202). Two-color flow cytometric contour plots showing the correlated expression of CD4 versus CD19 were derived from gated events with the forward and side light-scatter characteristics of intact lymphocytes. Staining cells in the presence of BD Horizon Brilliant Stain Buffer (Cat. No. 563794/566349; Middle Plots) or BD Horizon Brilliant Stain Buffer Plus (Cat. No. 563795; Right Plots) restores the major lymphocyte populations to their expected fluorescent staining patterns (dotted lines) when compared with cells stained without a Brilliant Stain Buffer (Left Plots). Flow cytometric analysis was performed using a BD LSRFortessa™ Cell Analyzer System.

Figure 1. Either BD Horizon Brilliant Stain Buffer or BD Horizon Brilliant Stain Buffer Plus should be used in multicolor immunofluorescent staining and flow cytometric analysis when using two or more BD Horizon Brilliant reagents. Whole human blood was stained with BD Horizon™ BV421 Mouse Anti-Human CD4 and BD Horizon™ BV711 Mouse Anti-Human CD19 (Top Plots) antibodies or BD Horizon™ BUV395 Mouse Anti-Human CD4 and BD Horizon™ BV605 Mouse Anti-Human CD19 (Bottom Plots) antibodies. Erythrocytes were lysed with BD FACS Lysing Solution (Cat. No. 349202). Two-color flow cytometric contour plots showing the correlated expression of CD4 versus CD19 were derived from gated events with the forward and side light-scatter characteristics of intact lymphocytes. Staining cells in the presence of BD Horizon Brilliant Stain Buffer (Cat. No. 563794/566349; Middle Plots) or BD Horizon Brilliant Stain Buffer Plus (Cat. No. 563795; Right Plots) restores the major lymphocyte populations to their expected fluorescent staining patterns (dotted lines) when compared with cells stained without a Brilliant Stain Buffer (Left Plots). Flow cytometric analysis was performed using a BD LSRFortessa™ Cell Analyzer System.


BD Horizon™ Brilliant Stain Buffer Plus

BD Horizon™ Brilliant Stain Buffer Plus
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Product Details
Description
The BD Horizon Brilliant Stain Buffer Plus is a buffer formulated for the immunofluorescent staining of cells. This buffer was developed to have a reduced test volume for applications where total staining volume is a concern. Brilliant Stain Buffer Plus is as effective as BD Horizon Brilliant Stain Buffer (Cat. No. 563794/566349) in mitigating staining artifacts seen when using multiple BD Horizon Brilliant reagents. For optimal and reproducible results, either BD Horizon Brilliant Stain Buffer Plus or BD Horizon Brilliant Stain Buffer should be used anytime BD Horizon Brilliant™ dye-conjugated reagents are used. The BD Horizon Brilliant Stain Buffer Plus is compatible with the use of other fluorescent staining reagents conjugated with traditional fluorochromes, such as fluorescein, phycoerythrin or Alexa Fluor® dyes.
Preparation And Storage
Recommended Assay Procedures
Protocols for Multicolor Immunofluorescent Staining of Cells Using BD Horizon Brilliant Stain Buffer Plus
Multicolor Surface Staining of Human Cell Samples in Tubes or 96-Well Plates Using Individual Staining Reagents
1. Add 10 μL of BD Horizon Brilliant Stain Buffer Plus to all tubes or desired wells for the experiment
Note: The 10 μL amount of Brilliant Stain Buffer Plus per tube or per well does not depend on the final staining volume or amount of cells used per tube or number of BD fluorescent antibodies used in staining. Although written for use with human cells, these protocols can readily be adapted for analyzing mouse cells or cells from other species, for example, by staining mouse cells at 4°C rather than at room temperature (RT).
2. Add each fluorescent reagent at the recommended volume per test (eg, 5 μL or 20 μL). Proceed to Protocol 1, 2, or 3.
Note: If total volume of mixture from steps 1 and 2 does not meet or exceed 50 μL per test, bring total volume up to 50 uL by adding BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656).
Protocol 1 for Staining Whole Blood Samples in Tubes
a. Add 100 μL of human whole blood to each tube
b. Vortex tube contents
c. Incubate (30 min) the suspended cells protected from light at room temperature (RT)
d. Add 2 mL of BD FACS™ Lysing Solution (Cat. No. 349202; 10 min) or BD Pharm Lyse™ Lysing Buffer (Cat. No. 555899; 15 min) per tube and incubate protected from light at RT
e. Pellet cells by centrifugation (5 min) at 1400-1500 rpm
f. Aspirate supernatant; add 2-3 mL of stain/wash buffer, eg, BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656)
or BD Pharmingen™ Stain Buffer (BSA) (Cat. No. 554657)
g. Pellet cells by centrifugation (5 min) at 1400-1500 rpm
h. Aspirate the supernatant and resuspend cells in 500 μL of stain/wash buffer for flow cytometric analysis
Protocol 2 for Staining Peripheral Blood Mononuclear Cells or Bulk Erythrocyte-lysed Whole Blood Samples in Tubes
a. Add 100 μL of human cells to each tube
b. Vortex tube contents
c. Incubate (30 min) the suspended cells protected from light at room temperature (RT)
d. Add 2 ml of stain/wash buffer per tube
e. Pellet cells by centrifugation (5 min) at 1400-1500 rpm
f. Aspirate supernatant; add 2-3 mL of stain/wash buffer
g. Pellet cells by centrifugation (5 min) at 1400-1500 rpm
h. Aspirate the supernatant and resuspend cells in 500 μL of stain/wash buffer for flow cytometric analysis
Protocol 3 for Staining Peripheral Blood Mononuclear Cells or Bulk Erythrocyte-lysed Whole Blood Samples in 96-well Plates
Note: Although written for use with human cells, this 96-well plate-based protocol can readily be adapted for analyzing mouse cells or cells from other species.
a. Add 50 μL of human cells to each well
b. Incubate (30 min) protected from light at RT
c. Wash by adding 100 μL of stain/wash buffer
d. Pellet cells by centrifugation (5 min) at 1400-1500 rpm
e. Aspirate supernatant
f. Resuspend pelleted cells by adding 250 μL of stain/wash buffer
g. Pellet cells by centrifugation (5 min) at 1400-1500 rpm
h. Aspirate supernatant
i. Resuspend pelleted cells thoroughly with 150 μL stain/wash buffer by pipetting the suspended cells several times
j. Transfer well contents to tubes and add additional stain/wash buffer to the tubes as desired for flow cytometric analysis
Note: Alternatively, acquire samples for flow cytometric analysis from the plate directly.
Multicolor Intracellular Staining of Cell Samples in Tubes or 96-Well Plates Using Individual Staining Reagents
1. Add 10 μL of BD Horizon Brilliant Stain Buffer Plus to all tubes or desired wells for the experiment.
Note: The 10 μL amount of Brilliant Stain Buffer Plus per tube or per well does not depend on the final staining volume or amount of cells used per tube or number of BD fluorescent antibodies used in staining.
2. Add each fluorescent reagent at the recommended volume per test (eg, 5 μL or 20 μL).
Note: If total volume of mixture from steps 1 and 2 does not meet or exceed 50 uL per test, bring total volume up to 50 uL by adding BD Pharmingen Stain Buffer (FBS). For intracellular staining protocols that must keep cells permeabalized during staining use BD Perm/Wash™ Buffer (Cat. No. 554723).
Protocol 4 for Staining Intracellular targets in either tubes or 96 well plates
Note: For primary surface staining follow protocol 1-3 above as applicable. If the intracellular protocol requires the use of BD Perm/Wash Buffer during the staining and wash steps, use BD Perm/Wash Buffer in steps b, e and h, otherwise use BD Pharmingen Stain Buffer (FBS).
a. Permeabilize cells according to desired permeabilization protocol
b. Add 50 uL of appropriate staining buffer
c. Add one test volume of the mixture from steps 1 and 2 (staining reagents plus BD Horizon Brilliant Stain Buffer Plus)
d. Incubate at 4°C for 30-60 minutes in the dark.
e. Wash with appropriate wash buffer (1 ml/wash for staining in tubes and 250 µl/wash final volume for staining in microwell plates)
f. Pellet cells by centrifugation (5 min) at 1400-1500 rpm
g. Aspirate supernatant
h. Wash with appropriate wash buffer (1 ml/wash for staining in tubes and 250 µl/wash final volume for staining in microwell plates)
i. Pellet cells by centrifugation (5 min) at 1400-1500 rpm
j. Resuspend in BD Pharmingen Stain Buffer (FBS) prior to flow cytometric analysis.
Multicolor Staining of Human Cell Samples in Tubes or 96-Well Plates Using Cocktailed Staining Reagents
Instead of adding staining reagents individually to each tube or well of a 96-well plate, it may be desirable to add cocktailed staining reagents, ie, mixtures of two or more fluorescent staining reagents. The following protocol provides an example of how to prepare a "per test" 5-Color Fluorescent Antibody Cocktail that already contains BD Horizon Brilliant Stain Buffer Plus.
Human Samples: Pre-mixed Fluorescent Reagent Cocktails
For each multicolor test of cocktailed fluorescent reagents:
i) Add 10 μL of BD Horizon Brilliant Stain Buffer Plus per test
ii) Add each fluorescent reagent at the recommended volume per test (5 μL or 20 μL)
iii) Add BD Pharmingen Stain Buffer (FBS) (Cat. No. 554656) to bring the volume of each test to a minimum of 50 μL
iv) Mix reagents (especially after adding BD Horizon Brilliant reagents)
v) Store cocktail at 4°C protected from light if it is to be used later
Note: Protected from light, fluorescent reagent cocktails containing more than one Brilliant Violet and/or Brilliant Blue reagent are best used within 24 hours after preparation when stored at 4ºC or within 4 hours when stored at room temperature. However, when more than one Brilliant Ultraviolet (BUV) reagent is in the cocktail, it is best used within 2 hours after preparation irrespective of storage temperature.
For additional guidance, see Table 1 "Example of creating a 5-Color Fluorescent Antibody Cocktail containing 2 different BD Horizon™ Brilliant Violet (BV) Conjugates" at the end of this field.
Compensation and Setup
BD Horizon™ Brilliant Stain Buffer Plus can be used in single color compensation controls using cells. The buffer is compatible with BD® Compbeads, however, it has not been tested with compensation beads from other vendors. For the most accurate compensation, compensation controls created with either cells or beads should be exposed to BD Horizon™ Brilliant Stain Buffer for the same length of time as the corresponding multicolor panel. Additionally, the most accurate compensation will be created when BD Horizon™ Brilliant Stain Buffer is used in all compensation controls, including Brilliant polymer and non-polymer dyes.
Product Notices
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Alexa Fluor® is a registered trademark of Life Technologies Corporation.
- BD Horizon Brilliant Stain Buffer is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,575,303; 8,354,239.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
Data Sheets
Companion Products





Development References (7)
-
Acosta JR, Douagi I, Andersson DP, et al. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes.. Diabetologia. 2016; 59(3):560-70. (Methodology: Flow cytometry). View Reference
-
Andrés-Blasco I, Herrero-Cervera A, Vinué Á, et al. Hepatic lipase deficiency produces glucose intolerance, inflammation and hepatic steatosis.. J Endocrinol. 2015; 227(3):179-91. (Methodology: Flow cytometry). View Reference
-
Ingelman-Sundberg HM, Laestadius Å, Chrapkowska C, et al. Diverse effects on vaccine-specific serum IgG titres and memory B cells upon methotrexate and anti-TNF-α therapy in children with rheumatic diseases: A cross-sectional study.. Vaccine. 2016; 34(10):1304-11. (Methodology: Flow cytometry). View Reference
-
Leijten EF, van Kempen TS, Boes M, et al. Brief report: enrichment of activated group 3 innate lymphoid cells in psoriatic arthritis synovial fluid.. 2015; 67(10):2673-8. (Methodology: Flow cytometry). View Reference
-
McKay FC, Gatt PN, Fewings N, et al. The low EOMES/TBX21 molecular phenotype in multiple sclerosis reflects CD56+ cell dysregulation and is affected by immunomodulatory therapies.. Clin Immunol. 2016; 163:96-107. (Methodology: Flow cytometry). View Reference
-
O'Connor KS, Read SA, Wang M, et al. IFNL3/4 genotype is associated with altered immune cell populations in peripheral blood in chronic hepatitis C infection.. Genes Immun. 2016; 17(6):328-34. (Methodology: Flow cytometry). View Reference
-
Vinué Á, Andrés-Blasco I, Herrero-Cervera A, et al. Ink4/Arf locus restores glucose tolerance and insulin sensitivity by reducing hepatic steatosis and inflammation in mice with impaired IRS2-dependent signalling.. Biochim Biophys Acta. 2015; 1852(9):1729-42. (Methodology: Flow cytometry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.