How Spectral Flow Cytometry has Changed the Landscape of Fluorochrome Development for High-Dimensional Research
July 11, 2022
We have a deeper understanding of the cells now than ever before. With the increase in our understanding of cellular biology and of the molecular signatures of cells, the need for concomitant development of tools to interrogate them and understand their intricate relationships has also increased. Flow cytometry is a powerful technique for immunophenotyping and analyzing distinct cell populations. The advancement in dye development technologies has enabled an exponential growth in the number of available fluorochromes for flow cytometry in recent years.
Instrumentation capabilities have also increased alongside, expanding the number of lasers and detectors, as have the number of measurable parameters. But as the complexity of instruments and the availability of fluorochromes have increased, so have the challenges associated with designing flow cytometry panels. The complexity entailed designing panels by meticulously studying the range of the spectrum defined by the filters for each fluorophore and picking the right fluorophores to avoid fluorescence spillover and spread. Thus, fluorochrome development was largely restricted by the range of emission spectrum collected by the filters.
Spectral flow cytometry removed this barrier by affording the ability to explore the complete emission pattern of fluorochromes. The advent of spectral flow cytometry simply opened up the possibility to develop a large number of new dyes for larger multicolor panels. Here, we consider the differences between conventional and spectral flow cytometry and see how spectral flow cytometry changed the fluorochrome landscape.
Conventional flow cytometry versus spectral flow cytometry: what are the major differences?
Relationship between fluorochromes and detectors
The main difference between the two types of flow cytometry exists in the coverage of the spectrum by the detectors in the flow cytometers. In conventional flow cytometers, a fluorochrome’s emission spectrum is collected by a single detector. Only a portion of the fluorochrome’s emission is covered by the detector and the range of coverage is defined by an individual filter. Filters with the appropriate bandwidths detect the emission peaks of fluorochromes and avoid laser lines, areas of overlap and areas with no available fluorescent proteins. So, there is a 1:1 relationship between a fluorochrome and a detector in conventional flow cytometry. In order to cover the entire visible spectrum, additional detectors are added in conventional flow cytometers.
In Spectral flow cytometers, the entire spectrum of the fluorochrome’s emission is collected by multiple detectors or detection arrays. For each laser, the emitted light is split through prisms, filters or gratings and directed into individual detectors across the spectrum. In this case, a 1:many relationship exists between a fluorochrome and its detectors. Therefore, in general, the number of detectors in a spectral flow cytometer is higher than in conventional flow cytometers, although some sophisticated conventional flow cytometers, such as the BD FACSymphony™ A5 and BD FACSymphony™ A3 Cell Analyzers , have an equivalent number of fluorescent detectors as some spectral flow cytometers. The ability to collect more data points with spectral flow cytometers allows more granular dissection of signals, which could improve resolution between fluorochromes.
Compensation and spectral unmixing
In conventional flow cytometers, a portion of the emission spectrum of an individual fluorochrome is collected by an individual detector. But a single fluorochrome can be excited by multiple lasers; so the total fluorescence measured in a detector could be contributed by multiple fluorochromes. The process of compensation is used to subtract background signals from overlapping spectra in conventional flow cytometers.
Spectral flow cytometers measure the entire emission pattern of fluorochromes across the spectrum. Spectral unmixing algorithms afford the ability to distinguish the spectral profile of individual fluorochromes. This ability to resolve individual spectral signatures of highly overlapping fluorochromes (such as FITC and BD Horizon Brilliant™ Blue 515 [BB515]) increases the number of fluorochromes that can be used simultaneously in a spectral flow cytometer compared to a conventional flow cytometer. This also enables designing larger and more complex panels with spectral flow cytometers than conventional ones.
Development of new fluorochromes that have distinct spectral signatures
The ability of spectral flow cytometry to examine the entire spectrum has led to the development of new dyes with distinct spectral signatures that can be exploited to obtain better resolution and more granularity for high-parameter analyses. This inspired the innovation in the development of a new dye technology from BD Biosciences.
The newly introduced BD Horizon RealYellow™ 586 (RY586) Fluorochrome is the first in the family of BD Horizon RealYellow™ and RealBlue™ Reagents that leverages next-generation BD dye technology and AI-guided fluorochrome selection to optimize spectral positioning to deliver reduced spillover and optimal resolution when used with other fluorochromes. This enables its use alongside dyes that have high cross-laser excitation, such as phycoerythrin (PE), on spectral flow cytometers.
The PE fluorochrome is excited by both blue and yellow/green lasers and its emission profile shows distinct peaks on the UV, violet, blue and yellow-green detectors. The RY586 fluorochrome, however, has minimal cross-laser excitation off the blue and yellow/green lasers and shows a distinct peak at the yellow-green wavelength with lower emissions into the blue, violet and UV detectors.
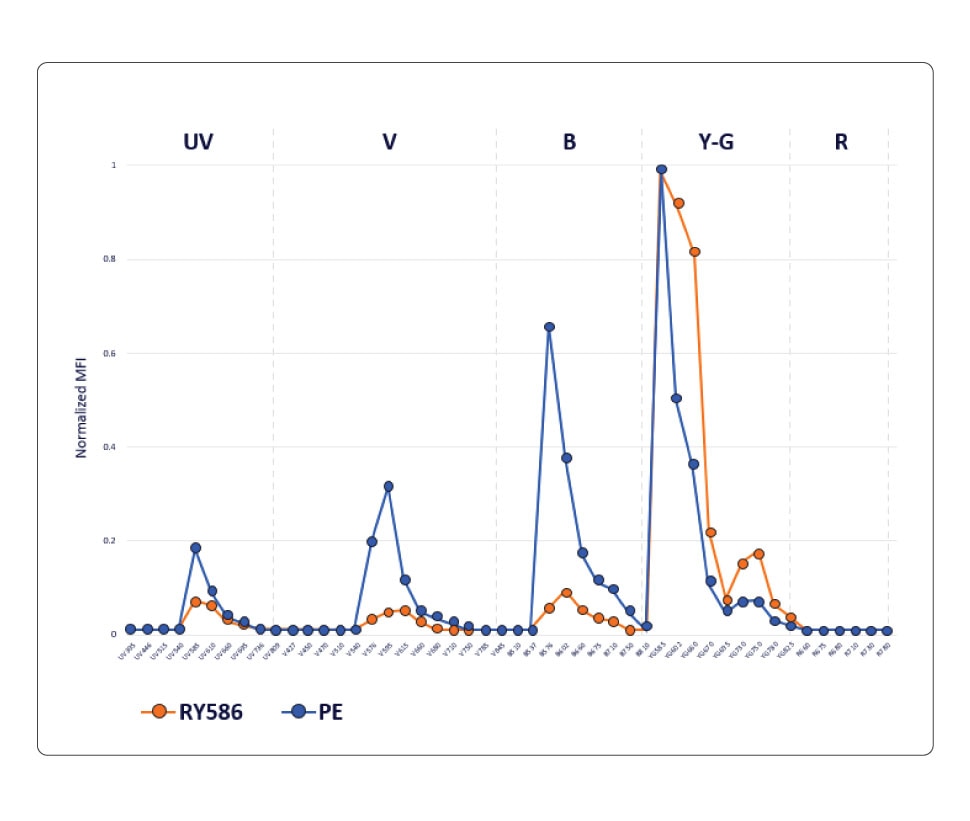 RY586 exhibits significantly less cross-laser excitation by the blue, violet and UV lasers, resulting in less spillover. This chart compares the normalized emission profile of PE and RY586, showing lower emission of RY586 into blue, violet and UV detectors.
RY586 exhibits significantly less cross-laser excitation by the blue, violet and UV lasers, resulting in less spillover. This chart compares the normalized emission profile of PE and RY586, showing lower emission of RY586 into blue, violet and UV detectors.
In a conventional workflow, where there is a 1:1 relationship between the detector and the fluorochrome, the overlap between PE and RY586 is too high to be resolved when these two fluorochromes are used in a panel. Therefore, only one of these fluorochromes can be used in a conventional flow cytometer.
However, the differences between PE and RY586 can be easily detected and resolved on a spectral flow cytometer, thus enabling the simultaneous use of these two fluorochromes. The ability to use RY586 along with PE on a spectral flow cytometer increases the number of parameters that can be measured. This is significant, especially in high-parameter analysis, where the addition of one more marker will allow for more granularity in results and can facilitate gleaning deeper scientific insights.
Through its ability to utilize the fluorochrome’s entire emission spectrum, spectral flow cytometry is indeed changing the fluorochrome landscape rapidly, paving the way for more innovative approaches and expanding the power of flow cytometry even further!
Check out the BD Horizon RealYellow™ 586 Reagents.
