-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- BD OMICS-One™ WTA Next Assay
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?
Multicolor Flow Cytometry Panel Design
Multicolor flow cytometry enables the simultaneous analysis of multiple markers at the single-cell level. By using fluorochrome conjugated antibodies that target specific cellular markers, researchers can analyze various cell characteristics like surface antigens and intracellular proteins. The design of a flow cytometry panel can be challenging and requires an understanding of several factors that can influence panel performance such as the fluorochromes and the instrument being used.
Optimizing Your Flow Cytometry Protocol
Flow Cytometry can be a complex process, requiring specialized expertise. Particularly, developing an optimized panel design for protein analysis requires careful consideration of factors such as marker identification and fluorochrome selection. At BD Biosciences, we are committed to guiding you through every step of this intricate process, ensuring that you achieve reliable and meaningful results.
Doing spectral? Check out our spectral flow cytometry resources.
When building assays, we are presented with hundreds of fluorochrome options and their different properties can make selection feel overwhelming. Check out our Fluorochrome Performance Guide with a handy cheat-sheet to help you prioritise clean fluorochromes and simplify your panel design.
BD Biosciences is backed by over 50 years of…

Panel Design for Flow Cytometry
In the following series of videos, you can learn the essential principles of how to design a flow cytometry panel from BD Biosciences scientists. Developing a panel design for protein analysis involves several steps to ensure the selection of antibodies that specifically recognize the target antigens with high affinity and minimal cross-reactivity, and includes:
- Identification of an experimental hypothesis
- Selection of the markers to be detected
- Understanding the setting on your flow cytometer
- Assigning the right fluorochromes to the identified markers
- Reviewing the panel
Tools like Optimized Multicolor Immunophenotyping Panels (OMIP) and the BD Panel Repository can also be used to check whether a flow panel that may meet your requirements already exists.
Step 1: Define your experimental hypothesis
To define your experimental hypothesis, you need to start by identifying the following:
- The biological information you are trying to obtain
- The population(s) of cells you wish to interrogate (i.e., the critical population)
- Which and how many markers you need
- Whether targets are found on the cell surface or intracellularly
It is important to fully understand the desired biological information as it dictates the subsequent steps needed for your flow cytometry experiment. Therefore, it is useful to learn all about the biology of your target cell populations and their markers from reputable sources before attempting to design your flow cytometry panel.

Step 2: Select the right marker
Next, you would need to identify which and how many markers you need to identify in the cell population of interest.
Pay attention to:
- Marker expression levels
- Primary antigen: Expressed at high densities, often defining lineages or cell subsets (e.g., CD4)
- Secondary antigen: Often expressed over a continuum, provides nuanced information about cellular states and phenotypes (e.g., CD45RA)
- Tertiary antigen: expressed at low density but is often critical for identifying rare or specialized cell populations within the sample (e.g., CD25)
- Marker co-expression, especially of dim markers
- The gating strategy needed to identify the population(s) of cells you wish to interrogate
Step 3: Know your flow cytometer
Understanding your instrument configuration will let you know how many markers and which fluorochromes your instrument can detect. This understanding is vital for your panel design.
Elements to consider include:
- Laser wavelength for excitation
- Number of detectors for each laser
- Filters available to detect the fluorochromes

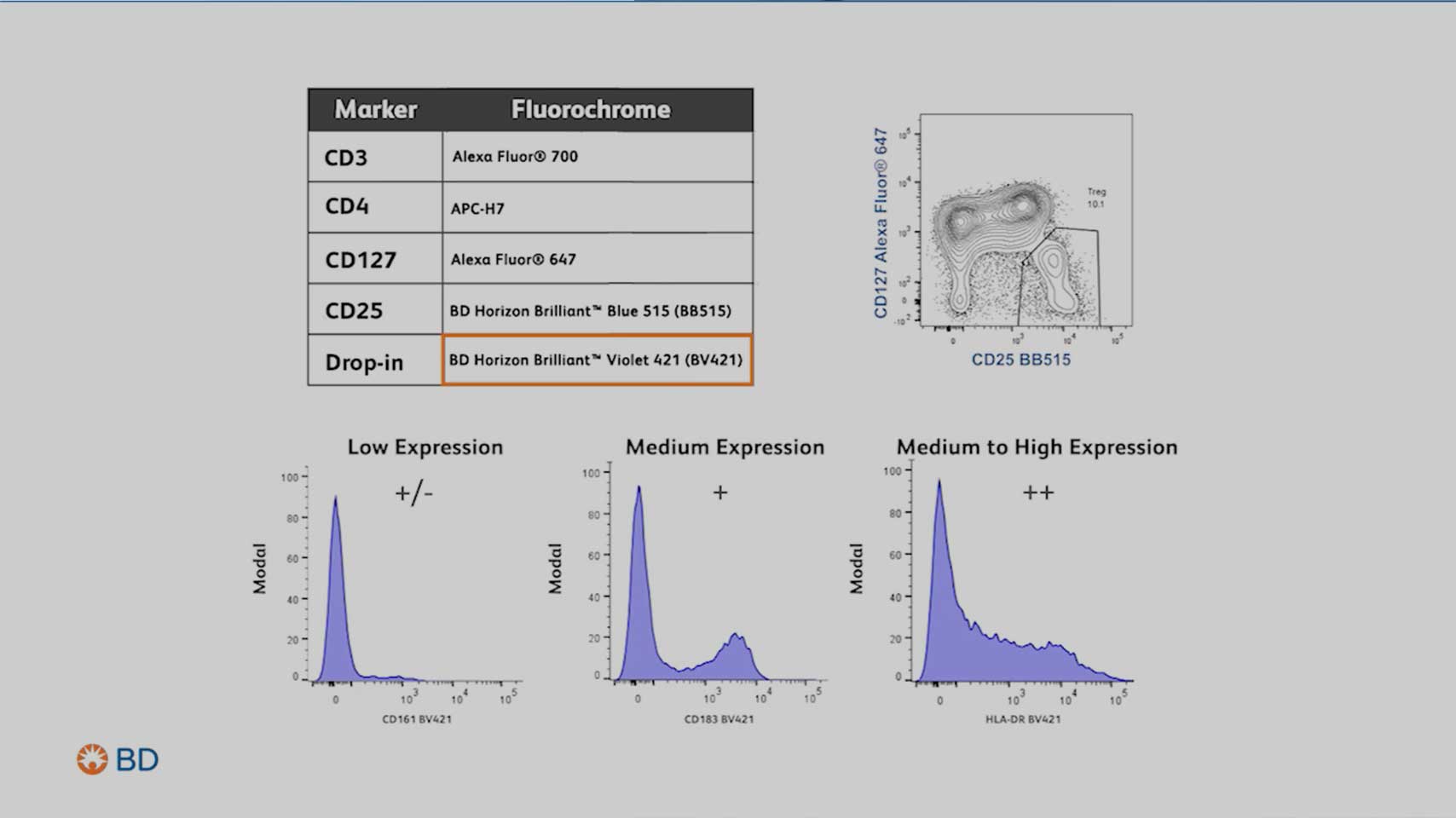
Step 4: Assign the right fluorochromes to the markers
Carefully select fluorochromes to resolve markers at all expression levels and minimize spectral overlap.
Consider using tools like a fluorochrome resolution ranking and a spectrum viewer to help assess:
- Cross laser excitation
- Fluorochrome spillover
Remember to pair bright fluorochromes with low expressing antigens and dim fluorochromes with high expressors. Keep in mind that spread only impacts the resolution of coexpressed markers.
Consider using laser-specific fluorochromes to reduce the complexity of the compensation and unmixing processes.

Your next steps
Discover insights, including thought leader opinions, breakthrough research and flow cytometry publication reviews. Visit our blog
Contact us today for a comprehensive evaluation of your panel design rationale to assess its effectiveness.
A Multicolor Flow Cytometry Journey - From Panel Design to Analysis
In this webinar we would like show you:
- Optimizing Fluorochrome Selection: Identify the best fluorochromes based on their performance in your experiments.
- Integrating Cell Biology & Panel Design: Explore the intersection of cell biology and panel design to unlock new research possibilities.
- Key Factors for Robust Panel Design: Understand the essential elements for creating reliable and effective mulicolor panels.

Speaker: Tim Schenkel, Research Application Consultant, BD Biosciences
Webinars
Blog Articles
E-learning Courses
Publications
- Mahnke YD, Roederer M. Optimizing a multicolor immunophenotyping assay. Clin Lab Med. 2007;27(3): 469–485, v. doi: 10.1016/j.cll.2007.05.002
- Mair F, Tyznik A. High-dimensional immunophenotyping with fluorescence-based cytometry: a practical guidebook. Methods Mol Biol. 2019;2032:1-29. doi: 10.1007/978-1-4939-9650-6_1
- Nguyen R, Perfetto S, Mahnke YD, Chattopadhyay P, Roederer M. Quantifying spillover spreading for comparing instrument performance and aiding in multicolor panel design. Cytometry A. 2013;83(3):306-315. doi: 10.1002/cyto.a.22251
- Roederer M. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry. 2001;45(3):194-205. doi: 10.1002/1097-0320(20011101)45:3<194::aid-cyto1163>3.0.co;2-c
Terms and Conditions
Panel iterations must be verified by a BD Applications Specialist. Valid only on panels designed by BD Biosciences in the last 2 months. Swaps that constitute more than a 20% change of the total number of reagents must be approved by BD.
Recommendations given by representatives of BD in the context of the creation and setup of customer-specific applications and assays and the composition of antibody panels for use in flow cytometry assays are provided in a diligent manner. However, the Customer acknowledges that (i) such recommendations should not be treated as a substitute for the Customer’s own examination, (ii) BD does not make any promises nor guarantees that recommendations are accurate and complete and will meet the Customer’s present or future needs or will produce positive or specific results and (iii) Customer remains solely responsible for validating and deciding on any such recommendations.
BD, therefore, accepts no liability whatsoever in connection with any such recommendation and/or any results generated on the basis of such recommendations and, to the fullest extent permitted by law, expressly disclaims any and all warranties and any liability in this respect. In particular, the Customer assumes sole responsibility for the validation, use, selection, and suitability of the recommendations for its needs and objectives.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
BD Flow Cytometers are Class 1 Laser Products