-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- BD OMICS-One™ WTA Next Assay
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?
Overview
The software that controls the BD FACSLyric™ Flow Cytometry System comprises two applications, BD FACSuite™ Clinical Application and BD FACSuite™ Application. Both applications provide an easy-to-use interface for data acquisition and analysis with the BD FACSLyric™ System.
The BD FACSuite™ Clinical Application supports BD IVD assays with predefined assay templates.
The BD FACSuite™ Application is designed for user-defined assays which need to be verified and validated by the user. It enables collaboration through the unique assay transfer feature, providing simple, reproducible and transferrable assay setup facilitating instrument-to-instrument and site-to-site standardization.

Functions included in both applications, including password protection, audit trail, electronic signatures and IQ/OQ procedures, assist in supporting 21 CFR Part 11 and electronic record integrity.
The BD FACSuite™ 1.6 Application offers innovative BD ElastiGate™ Technology, a machine learning enabled auto-gating algorithm, designed to reduce error-prone manual gating, boost standardization and enhance gating of populations.
FEATURES
The BD FACSuite™ Clinical Application provides a streamlined workflow, predefined assay templates for BD IVD assays, and comprehensive reporting
A streamlined workflow
BD® CS&T Beads are used to check cytometer performance and automatically make adjustments, ensuring consistent instrument setup from day to day. They are also used to perform comprehensive checks during system initialization and setup, including performance QC; verify system performance; and trigger the automated laser alignment process if required. This functionality is enabled by the BD FACSuite™ Clinical Application and ensures the entire setup process can be performed without manual intervention.
Predefined assay templates and comprehensive reporting
Assay modules with predefined templates optimized for use with specific BD IVD reagent kits can be installed on the BD FACSuite™ Clinical Application. These templates feature automated gating, calculations and report generation.

Predefined reports for BD IVD assays include laboratory, physician and supplemental reports and make reporting results fast and easy. Results can be easily exported to a laboratory information system (LIS).
View the application note for a Simplified Workflow using Automated Instrument Setup and Compensation on the BD FACSLyric™ Flow Cytometer.
APPLICATION
The BD FACSuite™ Application provides a streamlined workflow and automated instrument standardization enabling collaboration
A streamlined workflow
BD® CS&T Beads are used to perform comprehensive checks during system initialization and setup, including performance QC; verify system performance; ensure that detector settings are optimal; and trigger the automated laser alignment process if required. This functionality enabled by the BD FACSuite™ Application ensures the entire setup process can be performed without manual intervention.
Spillover value measurement is performed every 60 days using predispensed dried BD® FC Beads. Spillover values are maintained within the software and updated automatically with PMTV changes.
Standardization and collaboration
Universal setup is an automated system that establishes and maintains instrument MFI target values on a single instrument using the BD FACSuite™ Application, BD® CS&T Beads and BD® FC Beads, allowing for reproducible and consistent results within and between BD FACSLyric™ instruments over time.
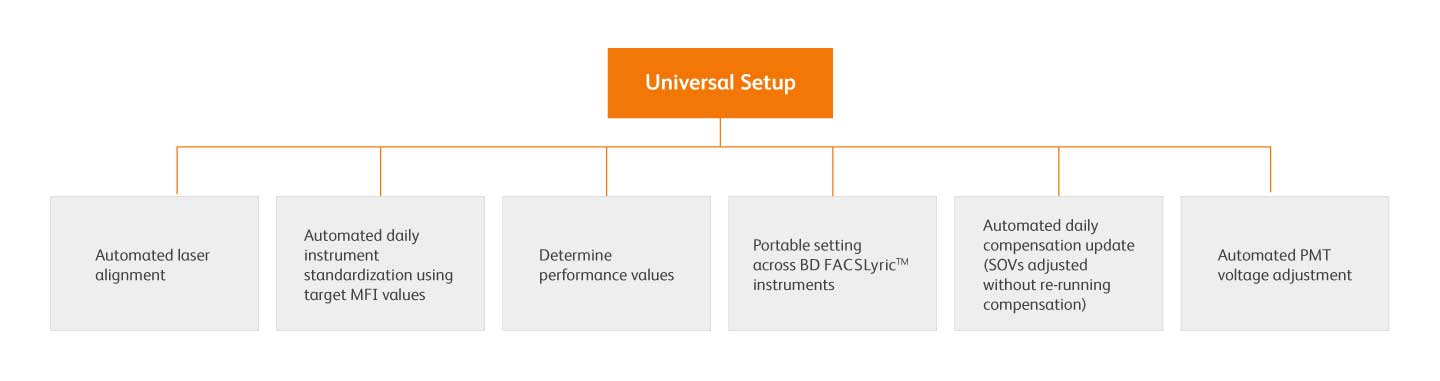
These settings can be easily transferred from instrument-to-instrument via the BD FACSuite™ Application assay export and transfer feature automating standardization and enabling collaboration across sites.
Highly reproducible results between BD FACSLyric™ instruments drive standardization
| BD FACSLyric™ System Variance (CVs) | |||||
| 15 BD FACSLyric™ Systems: Lyse/Wash settings and BD® FC Beads | |||||
| Blue Laser | %CV | Red laser | %CV | Violet laser | %CV |
| FITC | 4.2 | APC | 5.3 | BD Horizon™ V450 | 11.1 |
| PE | 4.4 | APC-Cy7 | 4.1 | BD Horizon™ V500 | 11 |
| PE-Cy7 | 4.8 | APC-H7 | 3.9 | BD Horizon™ BV605 | 13.6 |
| PerCP | 10.2 | APC-R700 | 4.2 | BD Horizon™ BV711 | 7.5 |
| PerCP-Cy5.5 | 7.9 | BD Horizon™ BV786 | 15.3 | ||
Between-instrument reproducibility of target MFI values on the BD FACSLyric™ Systems
Lyse/wash assay settings were imported across 15 instruments to show effects of standardization on beads. The CVs of the fluorescence intensity across all channels varies by less than 15.3%. Daily QC with one lot of BD® CS&T Beads was run on fifteen BD FACSLyric™ Cytometers. For each instrument, the PMTV gains were automatically adjusted to meet the target values. BD® FC Beads acquired on each BD FACSLyric™ Instrument. The MFI of positive populations was measured for all parameters across all instruments. The %CV is shown.
The data for this internal study were acquired using BD® FC Beads across 15 instruments. Greater between-instrument variability could be observed when running biological samples, when using non BD reagents or when comparing fewer instruments.
BD FACSuite™ Software Version 1.6
Request a demo.
*Required fields
Welcome to the BD FACSuite™ Application Academy, a learning portal dedicated to delivering up-to-date insight on the BD FACSuite™ Application. As we continue to make improvements, we want to keep you updated on all that the software has to offer. The BD FACSuite™ Application Academy provides valuable knowledge and is a great source of how-to and training videos all from a single online location. It is also a gateway to special on-demand webinars and special workshop events we offer to further guide you. Please visit often and keep your laboratory up to date on the BD FACSuite™ Application.
How to Use an Existing FCS File as a Training File to Create an ElastiGate Auto Gating Technology Assay
This video will demonstrate how to use an existing FCS file as a training file to create an ElastiGate Auto Gating Technology assay.
This video will demonstrate how to use a BD ElastiGate assay in a work list.
This video will demonstrate how to add training data to a BD ElastiGate assay.
Concatenation
Create a new FCS file by merging multiple files together
Create a custom analysis report
Create a gated plot directly from the parent gate
Define gate locations in one entry and apply those locations to other entries
Align your own lasers using BD® CS&T Beads
Choose the order of viewed populations in a plot
Remove setup data that is no longer needed to improve software performance
Customize the number of SIT flushes between tubes
BD FACSLyric™ Flow Cytometers are Class 1 Laser Products.
![]() BD® CS&T Beads and BD FACSLyric™ Flow Cytometer with the BD FACSuite™ Clinical and BD FACSuite™ application are in vitro diagnostic medical devices bearing a CE mark.
BD® CS&T Beads and BD FACSLyric™ Flow Cytometer with the BD FACSuite™ Clinical and BD FACSuite™ application are in vitro diagnostic medical devices bearing a CE mark.
Cy is a trademark of Global Life Sciences Solutions Germany GmbH or an affiliate doing business as Cytiva.