Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


.png)

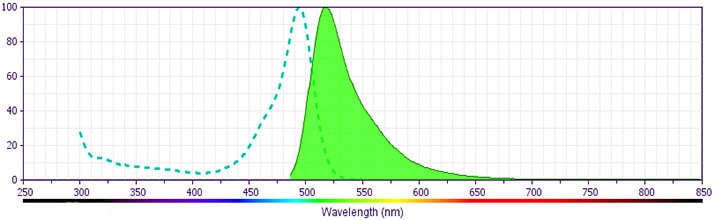
Detection of intracellular rat IgM in an antibody-secreting hybridoma cell line. Cells were fixed, permeabilized, and stained according to the method described below using FITC-conjugated G53-238 mAb (filled histogram) or the matched istotype control, FITC-conjugated MOPC-21 mAb (open histogram, Cat. No. 554679). Flow cytometry was performed on a BD FACSCalibur™ flow cytometry system.
.png)

BD Pharmingen™ FITC Mouse Anti-Rat IgM
.png)
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
FITC-conjugated G53-238 mAb may be used as a primary or secondary reagent in immunofluorescent staining. For flow cytometric detection of intracytoplasmic IgM, please refer to the following protocol.
IMMUNOFLUORESCENT STAINING OF INTRACELLULAR IMMUNOGLOBULIN (Ig) PROTOCOL
1. Prepare a single-cell suspension and determine cell number.
2. Suspend cells in staining buffer (PBS + 2% FBS + 0.1% Sodium Azide) at 2 x 10e7 cells/ml and transfer to U-bottom microwell plates in 50 µl/well for immunofluorescent staining.
Note: The BD Pharmingen. Stain Buffer with FBS (Cat. No. 554656) is effective for use as a staining buffer in this protocol.
3. Block Fcγ receptors by adding Rat BD Fc Block., purified anti-rat CD32 mAb D35-485 (Cat. No. 550270/550271) in 50 µl of staining buffer to each well.
4. Incubate 5 minutes on ice.
5. Add 200 µl of staining buffer/well and resuspend cells. Centrifuge at 250 x g for 5 minutes and aspirate supernatant.
6. Block surface Ig with purified G53-238 mAb (Cat. No. 553885) by adding 1.0 µg per sample in 50 µl of staining buffer/well.
Note: Surface markers may be stained during this step as described in the "Immunofluorescent Staining of Mouse and Rat Leukocytes for Flow Cytometry" in the Technical Protocols section of our website at http://www.bdbiosciences.com/pharmingen/protocols/Mouse_and_Rat_Leukocytes.shtml
7. Incubate 15 minutes on ice.
8. Wash 2x as described in Step 5.
9. Resuspend cells in 100 µl of BD Cytofix/Cytoperm. intracellular staining buffer (BD Cytofix/Cytoperm. Kit, Cat. No. 554714) per well.
10. Incubate 30 minutes at room temperature.
11. Wash 2x with 200 µl of 1 x Perm/Wash buffer (provided in the BD Cytofix/Cytoperm Kit) per well. Centrifuge at 250 x g for 5 minutes and aspirate supernatant between washes.
12. Stain intracellular Ig by adding ≤ 1 µg of FITC-conjugated G53-238 mAb in 50 µl of 1 x Perm/Wash buffer/well.
Note: Other antibodies recommended for staining of intracellular markers may be added during this step as described in Step 12.
13. Incubate for 30 minutes at room temperature.
14. Wash 2x as described in Step 11.
15. Resuspend and transfer samples in 100 µl of staining buffer to tubes appropriate for analysis with a flow cytometer. Bring volume in each tube to 400 µl with staining buffer.
16. Analyze samples on a flow cytometer.
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
The G53-238 antibody reacts specifically with rat IgM monomers and pentamers. It does not react with other Ig isotypes. G53-238 antibody has not been shown to stimulate B-cell proliferation.
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.