Your Path to IVDR: Simplify Your Lab Workflow with BD’s Comprehensive Solution
April 29, 2024
Treatment and disease monitoring are crucial to deliver the best possible patient care.
Laboratories have to produce consistent, timely and quality results to clinicians. However, laboratories are also facing several challenges, and addressing them is crucial for efficient operations and to obtain accurate results.
Workload management and resource constraints are considered important situations laboratories today have to cope with. Moreover, labs must ensure compliance with regulations such as IVDR and maintaining accreditation (e.g. ISO 15189 certification) and to do this, continuous monitoring and effort is required: e.g. meeting documentation requirements, reviewing clinical experience and taking appropriate corrective actions to name just a few of the conditions under Article 5(5) of IVDR.
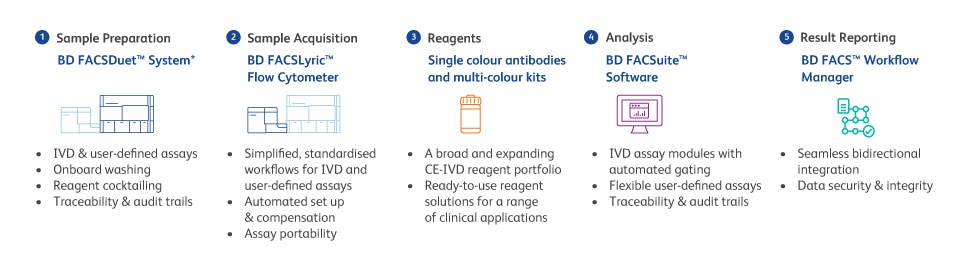
We offer a complete integrated clinical flow cytometry solution that simplifies and automates processes, with traceability and electronic audit trails, helping to drive compliance, automation, standardisation and quality.
Our comprehensive portfolio of clinical reagents offers flexibility in terms of ready to use kits (which reduces potential mistakes), but BD also offers an expanding portfolio of single colour CE-IVD reagents.
Our clinical flow cytometer and sample preparation system provides a walk away solution that may help a flow cytometry lab move forward on the path to IVDR.
To reduce complexity of assay transfer, the assay portability feature, along with the audit features included in the software, help in streamlining the documentation process.