-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- Norway (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
BD Pharmingen™ Recombinant Human IFN-γ

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Product Details
Description
Interferon-γ (IFN-γ) is a potent multifunctional cytokine which is secreted by activated NK cells and CD4+TCRαβ+, CD8+TCRαβ+, and TCRγδ+ T cells. IFN-γ exerts its biological effects through specific binding to a single class of high affinity receptors. In addition to its antiviral effects, IFN-γ can upregulate a number of lymphoid cell functions including the antimicrobial and antitumor responses of macrophages, NK cells, and neutrophils. In addition, IFN-γ can exert strong regulatory influences on the proliferation, differentiation, and effector responses of B cell and T cell subsets. These influences can involve IFN-γ's capacity to boost MHC class I and II expression by antigen-presenting cells as well as direct effects on B cells and T cells themselves. Human IFN-γ is a 14 - 18 kD multiple glycosylated form protein containing 143 amino acid residues. Recombinant human IFN-γ (Cat. No. 554616) is supplied as a frozen liquid comprised of
0.22 μm sterile-filtered aqueous buffered solution containing bovine serum albumin, with no preservatives. Human IFN-γ is ≥ 95% pure as determined by SDS-PAGE and an absorbance assay based on the Beers-Lambert law. The endotoxin level is ≤ 0.1 ng/μg of human IFN-γ, as measured in a chromogenic LAL assay.
Preparation And Storage
Recommended Assay Procedures
Upon initial thawing, recombinant human IFN-γ (Cat. No. 554616) should be aliquoted into polypropylene microtubes and frozen at -80°C for future use. Alternatively, the product can be diluted in sterile neutral buffer containing not less than 1 - 10 mg/mL carrier protein, such as human or bovine albumin, aliquoted and stored at -80°C. For in vitro biological assay use, carrier-protein concentrations of 1 - 2 mg/mL are recommended. For use as an ELISA standard, carrier-protein concentrations of 5 - 10 mg/mL are recommended. Failure to add carrier protein or store at indicated temperatures may result in a loss of activity. The product should not be diluted to less than 25 μg/mL for long term storage. Carrier proteins should be pre-screened for possible effects in each investigator's experimental system. Carrier proteins may have an undesired influence on experimental results due to toxicity, high endotoxin levels or possible blocking activity.
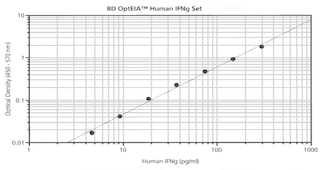
ELISA Standard: Recombinant human IFN-γ (Cat. No. 554616) can be useful as a quantitative standard for measuring human IFN-γ protein levels using sandwich ELISA with the purified NIB42 antibody (Cat. No. 551221) as a capture antibody and biotinylated 4S.B3 antibody
(Cat. No. 554550) as the detection antibody. To obtain linear standard curves, investigators may want to consider using doubling dilutions of recombinant human IFN-γ standard from 2000 - 15 pg/mL to be included in each ELISA plate. For measuring human IFN-γ in serum or plasma, investigators are highly encouraged to use the BD OptEIA™ Human IFN-γ ELISA Set (Cat. No. 555142 ) or BD OptEIA™ Human IFN-γ ELISA Kit II (Cat. No. 550612 ).
Bioassay: Investigators are advised that the Bioassay application is not routinely tested for this material and are highly encouraged to both titrate this material and include appropriate controls in relevant experiments. An activity range of 0.1 - 2.0 x 10^8 units/mg, encompassing an
ED50= 0.05 - 1.0 ng/mL, has previously been reported using A549 as indicator cells in an anti-viral bioassay, with a unit defined as the amount of material needed to stimulate a half-maximal response at cytokine saturation.
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Data Sheets
Companion Products

Development References (3)
-
Farrar MA, Schreiber RD. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993; 11:571-611. (Biology). View Reference
-
Green JA, Yeh TJ, Overall JC. Rapid, quantitative, semiautomated assay for virus-induced and immune human interferons. J Clin Microbiol. 1980; 12(3):433-438. (Biology). View Reference
-
Vogel S, Friedman R, Hogan M. Measurement of antiviral activity induced by interferons a, b, and g.. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, ed. Current Protocols in Immunology. New York: John Wiley & Sons; 2007:6.9.1-6.9.15.
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.