-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- BD OMICS-One™ WTA Next Assay
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?
Ease. Progress. Reliability.
When your flow cytometry laboratory flows, everyone and everything plays their part.
Your teams feel supported, your systems perform as designed and your results inspire clinical confidence.
Integrated solutions that link every step of the testing pathway can help you sail towards the future of clinical flow cytometry, from patient screening to patient monitoring.
What if you could improve patient care with greater results accuracy?
You and your flow cytometry laboratory are vital to enabling rapid, reliable clinical decision making.
Clinicians and patients alike trust you to provide consistent, timely and quality results, and the quality of those results is dependent on the standardisation of your laboratory workflow.1
However, standardisation isn’t always simple. Compliance challenges are compounded by the increasingly complex regulations, not to mention budget constraints and laboratory errors.
How can you ensure standardised laboratory practices to ensure positive patient outcomes?

What if you could increase lab efficiency with integrated solutions that support you and your team?
Workflow inconsistency, lack of standardisation, extended manual processes, staff shortages - your flow cytometry workflow undergoes an often overwhelming number of challenges that can influence your overall efficiency.
It may feel that to take one step forward, you are taking two steps back.
How can you implement efficient, standardised flow cytometry processes that support you and your team’s performance goals?
.png)
Solutions that flow, from patient screening to patient monitoring
While you’re committed to positively impacting patients’ lives, we’re committed to advancing your work through automation, standardisation and performance.
We’ve expanded our clinical flow cytometry family with integrated solutions to better support clinicians, patients and lab teams in oncohaematology, immunology, transplantation and transfusion.
Discover complete CE-IVD solutions for blood cancer diagnostics and immune assessment tests and learn how to address the needs of clinicians to better understand the immune system, immune response and minimal residual disease (MRD).
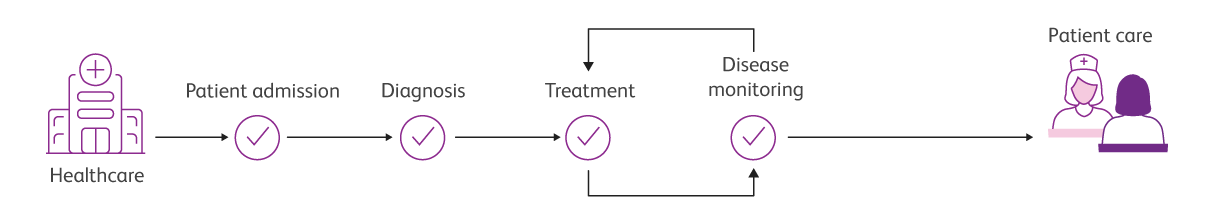
Integrated clinical solutions that flow, from sample to answer
Through the automation, standardisation and performance of our complete clinical laboratory solutions, you can achieve the efficiency and productivity you need to keep moving forward.
Standardisation and Automation
How can standardisation help you?
Standardisation is the consolidation of your laboratory systems and processes so that each task is performed in a way that benefits the whole workflow.
Standardisation and improvements in laboratory technologies can play key roles1 in:
- Enhancing results accuracy
- Helping prevent errors
- Increasing the quality of laboratory data
How can automation help you?
Automation helps to ensure that surges in patient specimen volumes do not impact quality, turnaround time and subsequently, the ability for clinicians to manage patient care effectively and safely.2-4
In particular, laboratory automation has been shown to save time by improving efficiency, reducing hands-on time and allowing you to reallocate staff time to more value-adding tasks.2
How does BD support?
By delivering timely and accurate results that inform clinical decisions, the integrated BD solution supports patient safety and positive health outcomes.3
The BD FACSDuet™ and the BD FACSDuet™ Premium Sample Preparation Systems paired with the BD FACSLyric™ Flow Cytometer deliver process standardisation by minimising error-prone steps while increasing workflow consistency and laboratory efficiency.
The BD FACSLyric™ combines simplicity, speed and automation to ease workload and improve productivity. This next-generation system enables flow cytometry workflow standardisation and collaboration through automation and unique assay portability capabilities.
Powered by the BD Infinicyt™ Software, certain applications within the integrated BD workflow provide automation in data analysis and reporting with EuroFlow™ Reference Databases, giving you stronger resources that prioritise reproducibility through standardisation.
The laboratory staff at the Nancy University Hospital in France decided to reorganise their flow cytometry practice with a full clinical solution. Now, data are directly transferred from the flow cytometers to their laboratory information system (LIS).
Learn more about the positive impacts BD integrated solution have had on efficiency and productivity by watching their story below.
Compliance - CE IVD
CE-IVD portfolio
In vitro diagnostic solutions within the BD Biosciences portfolio include flow cytometric immunophenotyping reagents for:
- Diagnosing, classifying and monitoring haematological disorders
- Monitoring human immunodeficiency virus (HIV)–infected individuals
- Characterising and monitoring immune deficiencies and autoimmune diseases
- Screening patients for suspicion of primary immunodeficiency
- Cell enumeration of residual leucocytes in blood products for transfusion
- Cell enumeration of CD34+ stem cells for haematopoietic stem cell transplants
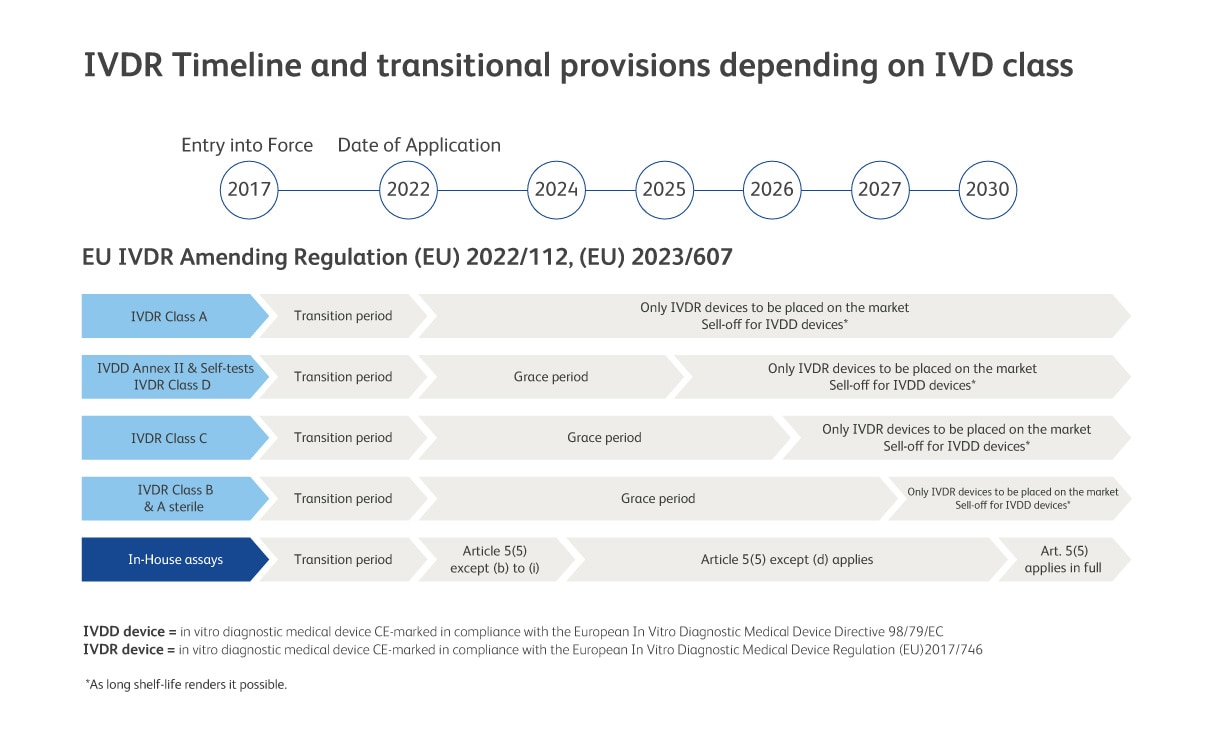
The European In Vitro Diagnostic Medical Device Regulation (IVDR) (EU) 2017/746 came into effect on 26 May 2017 and replaces the European In Vitro Diagnostic Medical Device Directive (IVDD) 98/79/EC. A global cross-functional team at BD Biosciences has been working to implement the new regulation in a seamless and compliant manner.
EU IVDR Amending Regulations (EU) 2022/112 and (EU) 2023/607 expands the scope of the grace period to certain devices and the abolishment of the sell-off provisions of IVDR. Consequently, devices placed on the market before the end of the transition period can be made further available on the market without a time restriction.
By your side through IVDR
We are committed to providing IVDR compliant solutions to continue delivering safe and effective IVD medical devices to serve you and your patients. We received our first IVDR product certification back in December 2020 and the majority of BD’s previous IVDD compliant devices are now IVDR compliant. We are continuing our efforts to achieve this for current and new products in our clinical portfolio.
Interested in solutions planned for IVDR compliance?
Data Analysis and Integration
How can you simplify complex data analysis?
You may feel that the more parameters you have when analysing flow cytometry data, the more challenges arise. With more analytic parameters
comes more opportunity for user variability, which may be costing your laboratory valuable time and resources.
How can automated analysis and reporting features help you?
Implementing automation solutions can help you increase consistency and reproducibility while decreasing inter-operator variability.
Automated analysis and reporting software features can:
- Support standardisation and reproducibility
- Help reduce inter-operator variability with less manual steps in analysis
- Boost workflow efficiency with automated reporting, integrated bidirectional LIS connection and less error-prone steps
How does BD support?
BD Biosciences offers a unique set of clinical software and informatic tools that drives laboratory workflow efficiency, even through complex multiparametric data analysis, and inspires confidence in reported results.
The BD FACSuite™ Clinical Application, the software behind the CE-IVD assays on the BD FACSLyric™ Flow Cytometry System, enables automated gating and analysis for a standardised, efficient and less error-prone workflow.
The BD FACSuite™ Application offers streamlined workflow features to support setting up user-defined assays with unique and simple electronic assay portability that enables sharing of user-defined protocols within and between institutions.
The BD Infinicyt™ Software was designed for multiparametric analysis of complex data, providing CE-IVD manual and automated data analysis and reporting when working with the intended uses of the appropriate BD CE-IVD panels for a comprehensive evaluation of pathological and rare cells to achieve the appropriate sensitivity.
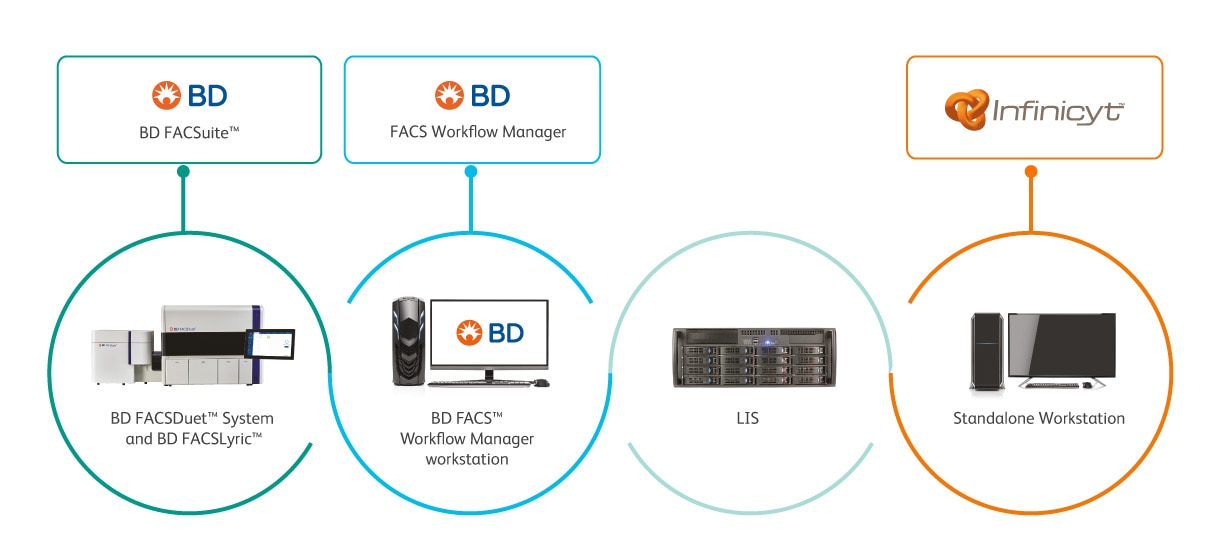
The BD FACS™ Workflow Manager is the BD Middleware solution, allowing bidirectional communication of test and patient data between the BD Clinical Solution Instrumentation, including the BD FACSLyric™ Flow Cytometry System and the BD FACSDuet™ System, standalone workstation and hospital and/or laboratory information system (HIS/LIS). By minimising manual tasks through features such as data storage, query and reporting on full testing history with original data files, it can help you reduce errors in results reporting.
Flexibility
How do you retain flexibility while prioritising standardisation?
Standardisation and flexibility are not mutually exclusive. There are solutions that can keep you and your teams agile while still adhering to standardised protocols in the laboratory.
With the help of automated solutions and software across the clinical flow cytometry workflow, you can enjoy the freedom of flexibility within a structured system.
How can BD support?
BD offers a large selection of IVDR compliant and RUO (GMP) single-colour reagents that give you the flexibility of setting up user-defined assays according to your needs. Select among many different fluorochromes, from dim to bright, depending on your panel.
The BD FACSuite™ Application offers streamlined workflow features to support the development of user-defined assays. Unique and simple electronic assay portability enables sharing of user-defined protocols within and between institutions to simplify and standardise instrument setup within your lab and between labs, making collaboration effective and efficient. With one BD FACSuite™ software menu for user-defined assays in the BD FACSuite™ Application and CE-IVD assays in the BD FACSuite™ Clinical Application, you can onboard your teams with less training effort.
The BD FACSDuet™ Sample Preparation System integrated with the BD FACSLyric™ Flow Cytometer, with the addition of automated on-board washing and on-board centrifugation, further enhances lab’s efficiency, with the flexibility needed for workflows of user-defined assays . The onboard automated antibody cocktail preparation reduces hands-on-time and eliminates error prone steps due to manual pipetting.
The BD Infinicyt™ Software provides flexibility in multiparametric CE-IVD data analysis when working with the intended uses of the appropriate BD CE-IVD panels, but also provides flexibility in the analysis of user-defined assays:
- Manual analysis: As an aid in the diagnosis and monitoring of oncohaematological diseases and screening of suspected primary immunodeficiencies (CE-IVD analysis) and in the analysis of any user-defined assays.
- BD Infinicyt™ profiles: Analysis templates in which the user can save the diagram configuration, reference images, analysis strategies and report configurations, among other options, to be used for specific analyses
- File merge tool: Allows the user to combine different data files and merge their information into a single file
Oncohaematology
BD offers a complete CE-IVD solution to cover all the needs of the clinical oncohaematology laboratory, from sample preparation to automated reporting, including the support of our highly specialised technical team.

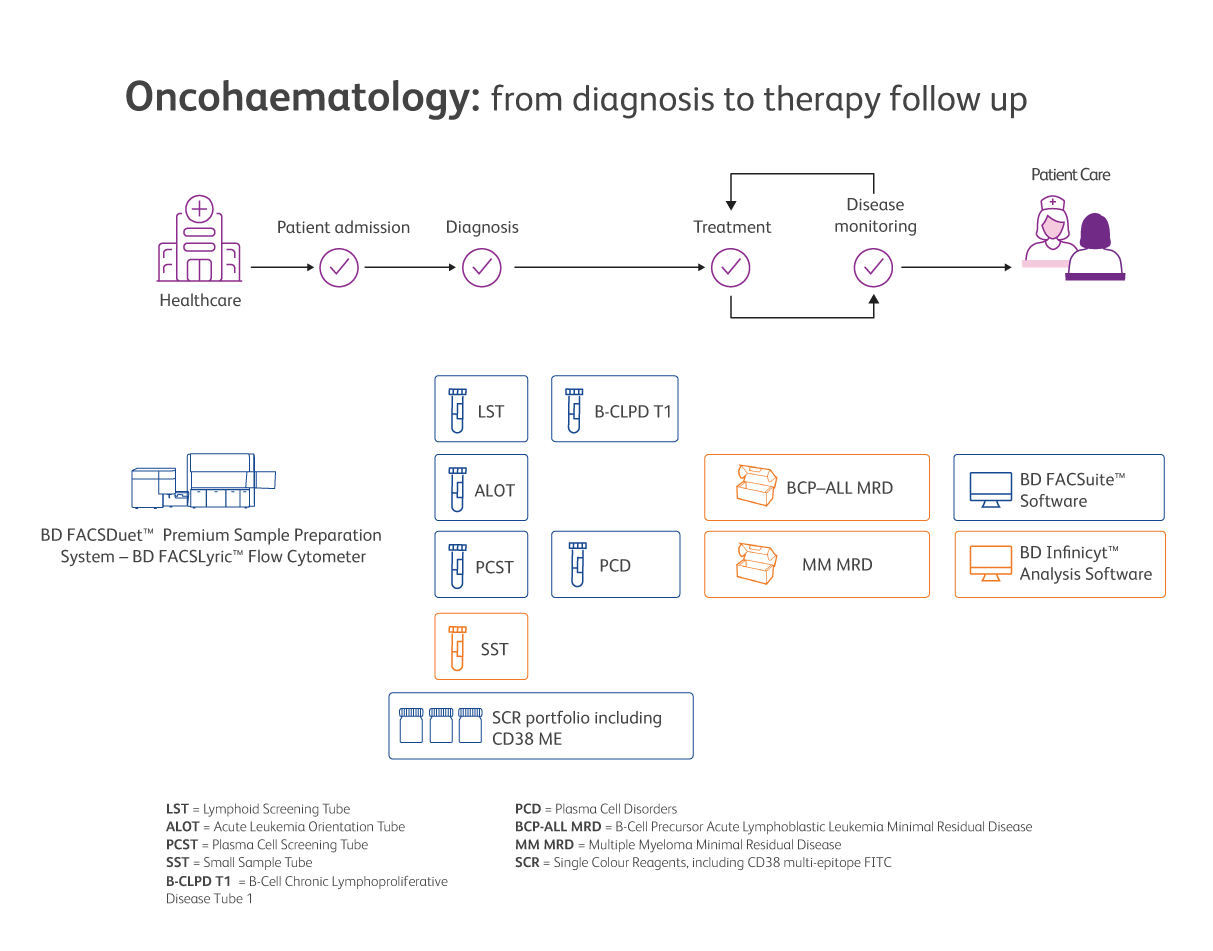
Available BD Biosciences solutions are presented on this image. Always check before running an IVD assay if additional validation is required.
Flow Through Blood Cancer Diagnosis
BD offers a CE-IVD flow cytometry solution to aid lab managers and clinicians in the diagnosis and monitoring of oncohaematological diseases.
Built on the research and validation work of the EuroFlow™ Consortium on the characterisation of haematological malignancies for improved accurate diagnosis and monitoring,5 our BD OneFlow™ Solution as well as minimal residual disease (MRD) detection with the Next Generation Flow™ (NGF*) method support the standardisation of immunophenotyping of haematological malignancies, lab efficiency and accuracy of results.
The BD OneFlow™ Solution is a growing comprehensive set of ready-to-use dried reagents (LST, B-CLPD T1, PCST, PCD and ALOT), protocols and assay templates to reproducibly set up the flow cytometer and stain, acquire and analyse patient specimens for immunophenotyping of normal and aberrant cell populations.
Our (NGF*) MRD detection solution for Multiple Myeloma and BCP-ALL, respectively, is an advanced method of multicolour flow cytometry that rapidly alerts clinicians if the disease is still present or if there is a sign of recurrence. The solution allows the simultaneous analysis of several phenotypic markers on up to 107 cells, showing detection limits comparable to the most sensitive molecular techniques.
CE-IVD single-colour antibodies complete the oncohaematology offer by contributing to consistency and providing flexibility.

*NGF: Next Generation Flow™. Next Generation Flow™ is a trademark or registered trademark of BD.
Immunology
BD offers CE-IVD solutions for assessing and monitoring secondary immune deficiencies and for screening patients with suspicion of primary immunodeficiencies.

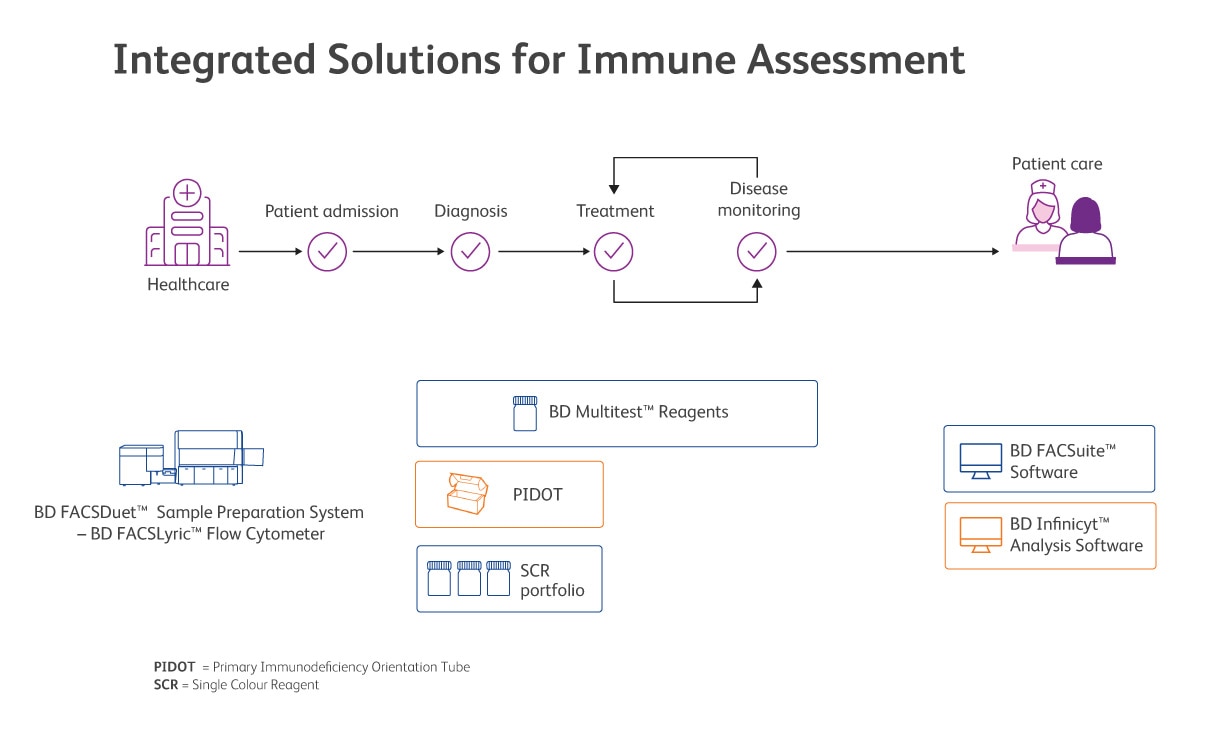
Available BD Biosciences solutions are presented on this image. Always check before running an IVD assay if additional validation is required.
Assessing the Immune System for Immune Deficiency
Primary immunodeficiencies (PIDs) occur when some parts of the immune system are absent or deficient. PIDs are usually congenital, deriving from hereditary genetic defects.
Secondary immunodeficiencies (SIDs) are induced by extrinsic factors in an immune system that is intrinsically competent. It is important to distinguish PIDs and SIDs, therefore having standardised solutions for immune deficiency analysis has a clinical impact.
Flow through immunophenotyping workflows
BD offers CE-IVD reagent solutions for assessing the immune system to enable you to accurately determine lymphocyte subpopulations in different clinical context, from screening for lymphoid PIDs to extrinsically induced SIDs.
BD Multitest™ Reagents with optional BD Trucount™ Tubes offer a choice of different combinations for identification and enumeration (percentage and absolute counts) of mature lymphocyte subsets. Monitoring of HIV patients, characterisation and monitoring some forms of immunodeficiency and autoimmune diseases are the clinical applications.
Complete the integrated solution with the BD FACSDuet™ Sample Preparation System, allowing a complete automated workflow from sample to answer to help reduce hands-on time and error-prone steps.
The EuroFlow™ Consortium has developed a standardised algorithm for flow cytometric screening of patients with suspicion of PID that can guide and prioritise further diagnostic modalities and clinical pathways.6,7 The use of our PID Orientation Tube (PIDOT), based on the scientific validation work of the EuroFlow™ Consortium, enables standardised flow cytometric screening of patients with suspected PIDs, detecting more than 20 leucocyte sub populations. Using the Next Generation Flow™ (NGF*) approach, greater process standardisation and higher sensitivity as compared to classical flow cytometry can be achieved. This includes the usage of BD Infinicyt™ Software and the PIDOT EuroFlow™ Database, to enable automated data analysis and reporting of extended lymphocyte subsets, including age-specific reference ranges.
CE-IVD single-colour antibodies complete the immunology offer by contributing to consistency and providing flexibility in immune assessment.

*NGF: Next Generation Flow™. Next Generation Flow™ is a trademark or registered trademark of BD.
Transplantation and Transfusion
BD offers complete CE-IVD solutions to simplify stem cell and residual white blood cell enumeration, prioritising efficiency, sensitivity and reliability.
Flow through stem cell enumeration
An accurate measurement of CD34 is critical for dose requirement protocols in stem cell transplantation,8 as an incorrectly high result could lead to an infusate with less than the recommended threshold dose of CD34+ cells.
Flow cytometric enumeration of CD34+ haematopoietic stem cells and progenitor cells is an established method for the evaluation of bone marrow and stem cell grafts, and the development of the standardised International Society of Haematotherpy and Graft Engineering (ISHAGE) protocol further aids in flow cytometry–based measurement of CD34+ markers.9
The BD® Stem Cell Enumeration (SCE) Kit on the BD FACSLyric™Flow Cytometer is a CE-IVD solution with a simplified, acquisition-to-reporting and a standardised workflow that enables reliable enumeration of CD34+ stem cells for haematopoietic stem cell transplants while enhancing lab efficiency.
- Work with a proven CE-IVD solution that simplifies acquisition and gating following the ISHAGE guidelines for bone marrow, peripheral blood, cord blood and leukapheresis products.
- Use trusted BD Trucount™ Tube technology for determining absolute CD34+ and CD45+ counts and two-level clinically relevant process controls, providing accurate and reproducible results on a single platform.
- Enhance workflow efficiency by reducing compensation frequency and minimising hands-on time through an intuitive, guided workflow and faster, simpler assay setup.
- Minimise errors by automatically calculating relevant results.
Flow through blood transfusion
Blood transfusions are critical for saving lives, and the reliability of your residual white blood cell enumeration process can support safe blood transfusion products.
Enumerating residual leukocytes in leukoreduced blood products with reliable control processes is essential to ensure the quality of blood components.
The BD Leucocount™ Kit on the BD FACSLyric™ Flow Cytometer gives you full confidence in your residual white blood cell enumeration. This automated CE-IVD solution allows you to not only deliver sensitivity that aligns with leucoreduction guidelines but also streamline your workflow. Advance your capabilities with quality control you can rely on for safe blood transfusion products.
- Achieve reliable enumeration of residual white blood cells with a standardised CE-IVD assay
- Assess safety of blood transfusion products with sensitivity that aligns with leucoreduction guidelines
- Utilize BD Trucount™ Tube technology for an accurate and reproducible single platform assay
- Enable high throughput capability delivering results that seamlessly transfer through LIS integration
Case Studies and Testimonials
Learn how your peers have achieved greater lab efficiency, diagnostic accuracy and standardisation from sample prep to analysis, and how you can too.
Clinical workflow standardisation through automation
Learn more about how the integrated BD FACSDuet™ Sample Preparation System and BD FACSLyric™ Flow Cytometer System delivered process standardisation to the Royal London Hospital by minimising workflow error-prone steps, increasing workflow consistency and laboratory efficiency.
Discover BD OneFlow™ Solution
Read about a study conducted by the NIHR Newcastle In Vitro diagnostics Cooperative, the Health Economics Group at Newcastle University and Newcastle upon Tyne Hospitals NHS Foundation Trust aimed to assess the effects on safety, efficiency and costs for the clinical diagnostic laboratory to adopt the BD OneFlow™ Reagent Tubes for diagnosing chronic lymphocytic leukaemia.
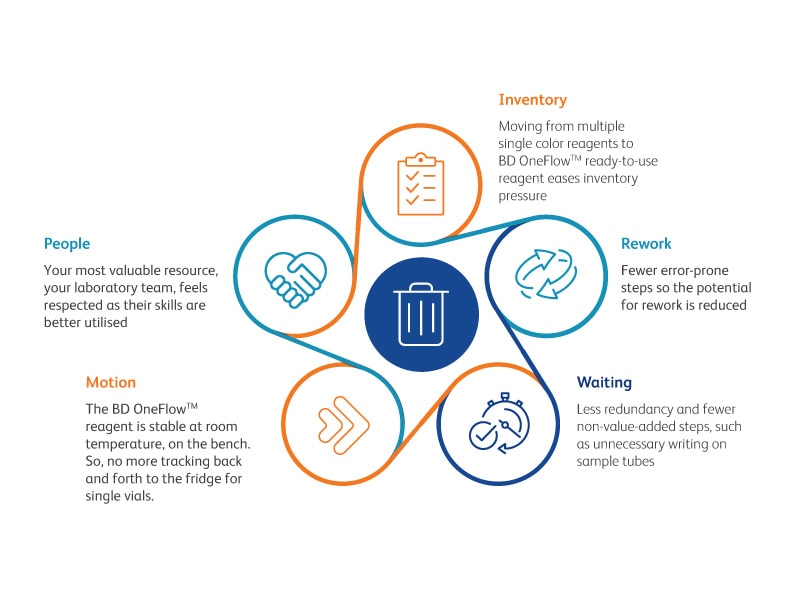
Discover how Standardization and Automation with BD OneFlow™ reduces Lean Wastes
Find out how, in the conditions compared in the study, the use of BD OneFlow™ assays on BD FACSLyric™ Flow Cytometer improved laboratory workflow and efficiency, by reducing hands on step by 69% and potential for analytical mistakes by 84% (error pone) to 92% (critical error prone).
Start a Conversation with a BD Clinical Product Expert
Find out how our complete line of clinical solutions can further your lab.
*Required fields
References
- World Health Organisation (WHO). Laboratory Quality Management System Handbook; 2011.
- Angeletti S, De Cesaris M, Hart JG, et al. Laboratory Automation and Intra-Laboratory Turnaround Time: Experience at the University Hospital Campus Bio-Medico of Rome. J Lab Autom. 2015;20(6):652-658.
- Howanitz J.H. and Howanitz P.J. Laboratory results. Timeliness as a quality attribute and strategy. Am J Clin Pathol. 2001;116(3):311-5
- Carraro P. and Plebani M. Errors in a Stat Laboratory: Types and Frequencies 10 Years Later. Clinical Chemistry. 2007;53: 1338-1342
- van Dongen JJM, Lhermitte L, Böttcher S, et al. on behalf of the EuroFlow Consortium (EU-FP6, LSHB-CT-2006-018708). EuroFlow antibody panels for standardised n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukaemia. 2012;26(9): 1908-1975. doi: 10.1038/leu.2012.120
- van Dongen JJM, van der Burg M, Kalina T,et al. EuroFlow-Based Flowcytometric Diagnostic Screening and Classification of Primary Immunodeficiencies of the Lymphoid. System. Front. Immunol (2019) 10:1271. Front. Immunol 2019:10:1271. doi: 10.3389/fimmu.2019.01271.
- Van Der Burg M, Kalina T, Perez-Andres M et al. EuroFlow-Based Flowcytometric Diagnostic Screening and Classification of Primary Immunodeficiencies of the Lymphoid. System. Front. Immunol (2019) 10:1271. doi:10.3389/fimmu.2019.00246.
- Langermayer I, Weaver C, Buckner CD, et al. Engraftment of patients with lymphoid malignancies transplanted with autologous bone marrow, peripheral blood stem cells or both. Bone Marrow Transplant. 1995;15(2):241-246.
- Sutherland DR, Nayyar R, Acton E, et al. Comparison of two single-platform ISHAGE-based CD34 enumeration protocols on BD FACSCalibur and FACSCanto flow cytometers. Cytotherapy. 2009;11(5):595-605.
BD Flow Cytometers and BD FACSDuet™ Sample Preparation System and BD FACSDuet™ Premium Sample Preparation System are Class 1 Laser Products.
![]()
BD FACSDuet™ Sample Preparation System, BD FACSDuet™ Premium Sample Preparation System, BD FACSLyric™ Flow Cytometer with the BD FACSuite™ Clinical and BD FACSuite™ Application, BD Infinicyt ™ analysis software, SST Panel, BCP-ALL MRD Panel, MM MRD Panel, PIDOT Panel and Cytognos CE-IVD Single Colour Reagents (including CD38-multi-epitope-FITC), BD Multitest™ CD8/CD38/CD3/HLA-DR and BD Multitest™ CD8/CD38/CD3/HLA-DR with BD Trucount™ Tubes (333184 and 333185) are in vitro diagnostic medical devices bearing a CE mark.
![]()
BD Multitest™ Reagents (excluding 333184, 333185), BD CE-IVD Single Colour Reagents, BD Leucocount™, BD OneFlow™ LST, BD OneFlow™ B-CLPD T1, BD OneFlow™ ALOT, BD OneFlow™ PCST, BD OneFlow™ PCD, BD® Stem Cell Enumeration Kit and BD Trucount™ Tubes are in vitro diagnostic medical devices bearing a CE mark and are CE certified by BSI Group The Netherlands B.V. (Notified Body Number = 2797).
The EuroFlow trademark is the property of the EuroFlow Consortium and cannot be reproduced or published without prior written permission from the EuroFlow coordinator (www.euroflow.org).