Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD Horizon™ Human T Cell Backbone Panel Kit
(RUO)




Figure 1. Optimal resolution of Human T cell subsets. Lysed whole blood from a healthy donor was stained with the Human T Cell Backbone Panel and acquired using a BD FACSymphonyTM A1 Cell Analyzer. Clear resolution of all the T cell subsets of interest was observed. Quadrant gates were applied from FMO controls. Figure 2. Recommended drop-ins for use with the Human T Cell Backbone Panel. A. Optional fluorochromes for use in the 5 open channels. B. Visualization of spread in recommended open channels on unstained sample and sample stained with the Human T Cell Backbone Panel components. The Human T Cell Backbone Panel introduces negligeable spread into the recommended drop-in channels. Spread is shown in detectors for BV421/V540, FITC/Alexa FluorTM 488/BB515, PE, APC/Alexa FluorTM 647 from a 12 color BD FACSLyricTM Cell analyzer and spread into BUV395 is shown from a BD LSRFortessaTM X-20 Cell Analyzer. Figure 3. Representative Total Spread Matrix (TSM) calculation for the recommended drop-in fluorochromes. Recommended drop-ins such as BV421, FITC, PE and APC are spatially separated and do not introduce spillover spread into each other. Figure 4. Backbone Panel complemented with surface and intracellular markers conventionally used to identify Human Treg cell subsets. Representative analysis of PMBCs, from a healthy donor, fixed and permeabilized with the BD Transcription Factor Buffer Set. Reagents were used at the recommended test size and staining followed the recommended BD Transcription Factor Buffer Set protocol. Clear resolution of all the subsets of interest was observed. Data were generated using a 4-laser BD FACSymphonyTM A1 Flow Cytometer.


BD Horizon™ Human T Cell Backbone Panel Kit
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Description
The Human T Cell Backbone Panel contains five individual vials of fluorochrome-conjugated antibodies against T cell core markers [CD3, CD4, CD8, CD45RA, CD197 (CCR7)] conventionally used to identify different maturational states of CD4+ and CD8+ T cells (naïve, central memory, effector memory, effector memory RA). The kit also contains the BD Horizon™ Brilliant Stain Buffer for optimal performance. The Human T Cell Backbone Panel is strategically designed to be complemented with 4-5 drop-in fluorochromes, ie, fluorescent reagents specific for biomarkers of choice, depending on instrument configuration, with minimal panel design effort. Assignment of fluorochromes for drop-ins have minimal impact on the resolution of the backbone panel. The fluorochromes used for the backbone panel have minimal resolution impact on the detectors allocated for drop-in fluorochromes and the specified fluorochromes available for drop-in do not impact each other. The Human T Cell Backbone Panel is compatible with intracellular stain and transcription factor analysis.
Preparation And Storage
Recommended Assay Procedures
For more information on the Human T Cell Backbone Panel, please visit our eBook at: www.bdbiosciences.com/content/dam/bdb/marketing-documents/BD-Horizon-Human-T-Cell-Backbone-Panel-EBook.pdf
• Development data was generated using 5 µl/test for each fluorescent reagent included in the Human T Cell Backbone Panel and 50 µl/test BD Horizon™ Brilliant Stain Buffer. Titration of reagents and assay optimization may be needed for custom cell samples and staining protocols.
• Brilliant Stain Buffer, provided in the kit components is recommend for use in all applications.
• For optimal resolution of the chemokine receptor CD197 (CCR7), prestaining with the anti-human CD197 BV711 antibody at 37˚ C for 10 minutes, before addition of the remaining antibodies and further incubation at RT for 30 minutes, is recommended
• BD Fc Block should be used when nonspecific Fc binding is of concern or drop-in reagents have mouse IgG2a isotypes.
• Brightness and spillover of the fluorochromes used in the backbone panel are minimally impacted by BD Cytofix/Cytoperm™ Buffer and BD Pharmingen™ Transcription Factor Buffer Set. BD Cytofix™ Fixation Buffer/BD Phosflow™ Perm Buffer III system is not recommended due to its harmful effects on PE and PE-Cy7 fluorochromes.
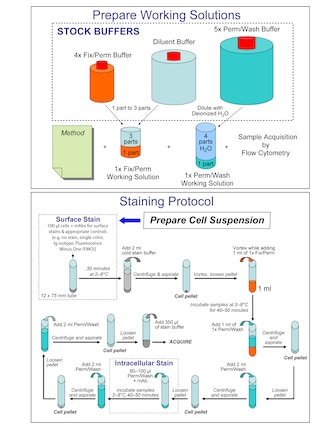
• A 3-step staining protocol is recommended for the use of the BD Horizon™ Human T Cell Backbone Panel together with intracellular drop-ins.
Stain surface markers → Fixation/Permeabilization → Stain intracellular markers
BD® CompBeads can be used as surrogates to assess fluorescence spillover (compensation). When fluorochrome conjugated antibodies are bound to BD® CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cells and BD® CompBeads to ensure that BD® CompBeads are appropriate for your specific cellular application.
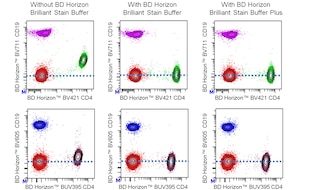
For optimal and reproducible results, BD Horizon ™ Brilliant™ Stain Buffer should be used anytime BD Horizon Brilliant™ dyes are used in a multicolor flow cytometry panel. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. The BD Horizon Brilliant Stain Buffer was designed to minimize these interactions. When BD Horizon Brilliant Stain Buffer is used in in the multicolor panel, it should also be used in the corresponding compensation controls for all dyes to achieve the most accurate compensation. For the most accurate compensation, compensation controls created with either cells or beads should be exposed to BD Horizon Brilliant Stain Buffer for the same length of time as the corresponding multicolor panel. More information can be found in the Technical Data Sheet of the BD Horizon Brilliant Stain Buffer (Cat. No. 563794/566349) or the BD Horizon Brilliant Stain Buffer Plus (Cat. No. 566385/568264).
Product Notices
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Please observe the following precautions: Absorption of visible light can significantly alter the energy transfer occurring in any tandem fluorochrome conjugate; therefore, we recommend that special precautions be taken (such as wrapping vials, tubes, or racks in aluminum foil) to prevent exposure of conjugated reagents, including cells stained with those reagents, to room illumination.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- This reagent has been pre-diluted for use at the recommended Volume per Test. We typically use 1 × 10^6 cells in a 100-µl experimental sample (a test).
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Warning: Some APC-Cy7 and PE-Cy7 conjugates show changes in their emission spectrum with prolonged exposure to formaldehyde. If you are unable to analyze fixed samples within four hours, we recommend that you use BD™ Stabilizing Fixative (Cat. No. 338036).
- An isotype control should be used at the same concentration as the antibody of interest.
- PE-Cy7 is a tandem fluorochrome composed of R-phycoerythrin (PE), which is excited by 488-nm light and serves as an energy donor, coupled to the cyanine dye Cy7, which acts as an energy acceptor and fluoresces maximally at 780 nm. PE-Cy7 tandem fluorochrome emission is collected in a detector for fluorescence wavelengths of 750 nm and higher. Although every effort is made to minimize the lot-to-lot variation in the efficiency of the fluorochrome energy transfer, differences in the residual emission from PE may be observed. Therefore, we recommend that individual compensation controls be performed for every PE-Cy7 conjugate. PE-Cy7 is optimized for use with a single argon ion laser emitting 488-nm light, and there is no significant overlap between PE-Cy7 and FITC emission spectra. When using dual-laser cytometers, which may directly excite both PE and Cy7, we recommend the use of cross-beam compensation during data acquisition or software compensation during data analysis.
- BD Horizon Brilliant Violet 510 is covered by one or more of the following US patents: 8,575,303; 8,354,239.
- BD Horizon Brilliant Violet 711 is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,227,187; 8,455,613; 8,575,303; 8,354,239.
- BD Horizon Brilliant Violet 786 is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,227,187; 8,455,613; 8,575,303; 8,354,239.
- BD Horizon Brilliant Stain Buffer is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,575,303; 8,354,239.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- This product is provided under an Agreement between BIOTIUM and BD Biosciences. This product, and only in the amount purchased by buyer, may be used solely for buyer’s own internal research, in a manner consistent with the accompanying product literature. No other right to use, sell or otherwise transfer (a) this product, or (b) its components is hereby granted expressly, by implication or by estoppel. This product is for research use only. Diagnostic uses require a separate license from Biotium, Inc. For information on purchasing a license to this product including for purposes other than research, contact Biotium, Inc., 3159 Corporate Place, Hayward, CA 94545, Tel: (510) 265-1027. Fax: (510) 265-1352. Email: btinfo@biotium.com.
- Alexa Fluor™ is a trademark of Life Technologies Corporation.
- Cy is a trademark of Global Life Sciences Solutions Germany GmbH or an affiliate doing business as Cytiva.
- Human donor specific background has been observed in relation to the presence of anti-polyethylene glycol (PEG) antibodies, developed as a result of certain vaccines containing PEG, including some COVID-19 vaccines. We recommend use of BD Horizon Brilliant™ Stain Buffer in your experiments to help mitigate potential background. For more information visit https://www.bdbiosciences.com/en-us/support/product-notices.
Companion Products




| Description | Quantity/Size | Part Number | EntrezGene ID |
|---|---|---|---|
| BV786 Mouse Anti-Human CD4 | 100 Tests (1 ea) | 51-9020198 | N/A |
| R718 Mouse Anti-Human CD8 | 100 Tests (1 ea) | 51-9020199 | N/A |
| PE-Cy7 Mouse Anti-Human CD45RA | 100 Tests (1 ea) | 51-9020508 | N/A |
| BV510 Mouse Anti-Human CD3 | 100 Tests (1 ea) | 51-9020507 | N/A |
| BV711 Mouse Anti-Human CCR7 (CD197) | 100 Tests (1 ea) | 51-9020511 | N/A |
| Brilliant Stain Buffer | 100 Tests (1 ea) | 51-9020509 | N/A |
Development References (3)
-
Hamann D, Baars PA, Rep MH. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997; 186(9):1407-1418. (Clone-specific: Flow cytometry). View Reference
-
Liechti T, Roederer M. OMIP-058: 30-Parameter Flow Cytometry Panel to Characterize iNKT, NK, Unconventional and Conventional T Cells.. Cytometry A. 2019; 95(9):946-951. (Clone-specific: Flow cytometry). View Reference
-
Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999; 401(6754):708-712. (Clone-specific: Flow cytometry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.