-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- BD OMICS-One™ WTA Next Assay
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?
Manufacturing and Quality Control in a GMP Environment
Cell therapy manufacturing and quality control (QC) in GMP environments has a number of challenges related to ensuring proper documentation, accurate and reproducible results, and scaling up for global manufacturing. To address these challenges, teams are investing in automated and easy-to-use flow cytometers, reagents manufactured in ISO 13485:2016 certified facilities required to follow Medical Device Current Good Manufacturing Practice (cGMP)*, software with features such as password protection, electronic signatures, automatic record keeping and audit trails that support 21 CFR Part 11, and lab information systems.
Optimizing manufacturing processes to reduce complexity and improve scalability requires the use of automated technologies. Flow cytometry solutions that are standardised across instruments allow data comparison and consistency of results across manufacturing sites and allow seamless tech transfers from research and development through to manufacturing.
*as stated in the Quality System Regulations (QSR) [U.S.Food and Drug Administration Reference 21CFR 820]
Explore how BD flow cytometry solutions can support key requirements for cell therapy manufacturing QC:
The manufacturing of cell therapy products requires testing at multiple stages and thus requires a significant amount of time and effort to ensure that the instrumentation and assays deliver accurate results. This drives interest in methods to improve efficiency of these workflows.
The BD FACSLyric™ Flow Cytometer enables efficiency and productivity with:
- Distinctive assay portability feature that allows easy and efficient transfer of assays across sites
- Automated analysis using the BD ElastiGate™ Autogating Algorithm in the BD FACSuite™ Application v1.6, which enables 1.8X faster analysis
- Fluorescence compensation required only every 60 days
- Flexibility for acquisition using a universal loader with 21 different loading options including multiwell plates
- Automated laser alignment
In addition, flow cytometry sample preparation can be automated using the BD FACSLyric™ Flow Cytometer integrated with the BD FACSDuet™ Sample Preparation System.
Benefits of this solution include:
- Physical integration of the sample preparation system with the flow cytometer to provide a complete end-to-end walkaway solution
- On-board, automated antibody cocktail preparation, washing, centrifugation and sample transfer that eliminates risk of errors due to manual pipetting
- Support for different types of blood collection tubes and a wide variety of reagent vials
Download the BD FACSDuet™ Premium Sample Preparation System brochure.
See below for data from a clinical lab demonstrating the reduction in hands-on time gained when using a BD FACSDuet™ System to automate a standard wash assay.
Reduce Operator Hands-on-time with the BD FACSDuet™ Premium System
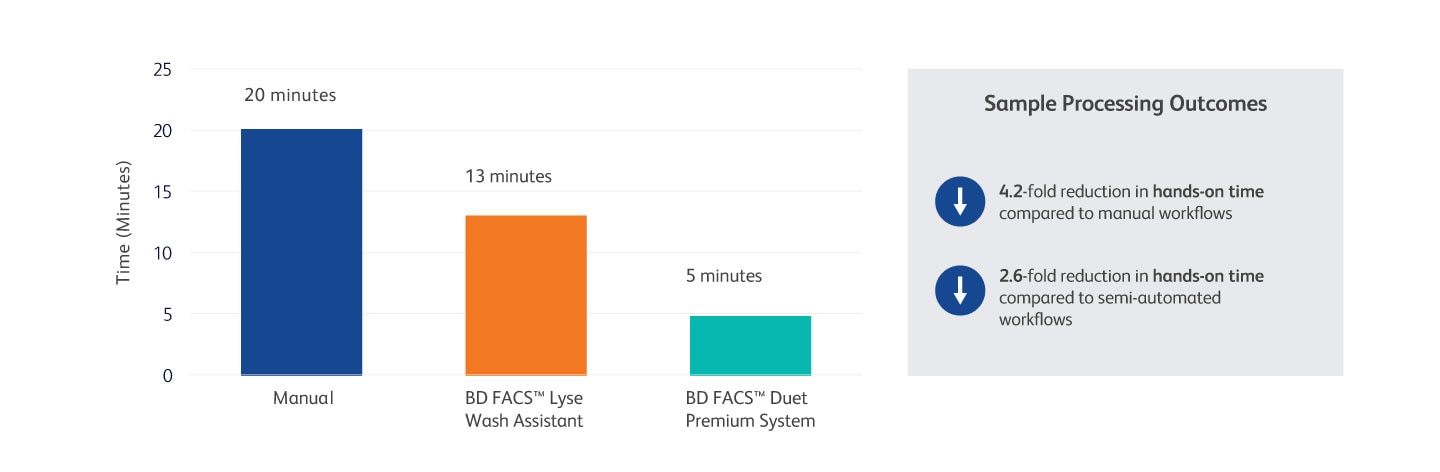
Hands-on time for manual, BD FACS™ Lyse Wash Assistant and BD FACSDuet™ Premium System three samples, 15-tube workflows
Study conducted at a U.S. clinical lab. Three workflows were compared for total processing time and hands-on time for manual, BD FACS™ Lyse Wash Assistant and BD FACSDuet™ Premium Sample Preparation System workflows. This process uses pre-cocktailed reagents and begins with pulling reagents out of the fridge for manual/BD FACS™ Lyse Wash Assistant workflows or loading specimens onto the BD FACSDuet™ Premium System. Three specimens were run with an SLW assay with five tubes per specimen for a total of 15 tubes per workflow.
Consistent Reagent and Instrument Performance and Streamlined AssayTransfer Enables Global Standardization
Ensuring consistent performance across the globe is critical for the distributed manufacturing and testing commonly found in cell therapy manufacturing as well as producing quality clinical research data. BD Biosciences has developed robust methods to make this easier.
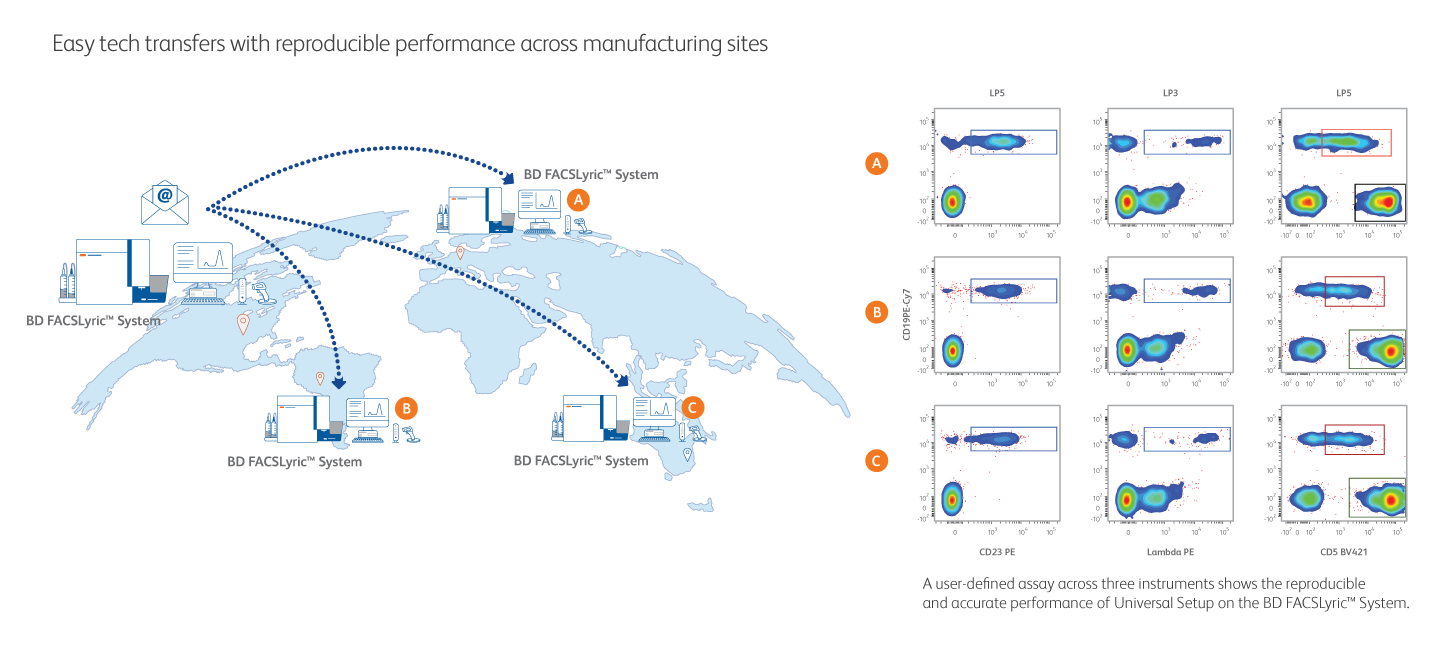
Streamlined Assay Transfer for Reproducible Performance Across Manufacturing Sites
Universal Setup on the BD FACSLyric™ Flow Cytometer allows transfer of user-defined assays from one instrument to another, supporting standardization of conditions for reproducibility across global manufacturing sites. This high-performance, highly sensitive flow cytometer demonstrates exceptional resolution and improved separation to make dim and rare populations easier to resolve.
The BD FACSDuet™ Sample Preparation System fully automates flow cytometry sample prep, including sample washing. The BD FACSDuet™ System adds traceability and automation to the preanalytical process.
BD Clinical Discovery Research Reagents are manufactured in an ISO 9001:2015 and ISO 13485:2016 certified manufacturing site to support your manufacturing QC assays. Using high quality reagents as part of your flow cytometry quality control helps to further standardize your manufacturing QC assay to give you confidence in the safety and integrity of your clinical research. High quality enables results that increase confidence in the data and that it can be safe for translating into clinical use in the future.
BD RUO (GMP) Reagents are manufactured in accordance with current Good Manufacturing Practices. Unit-sized preformulated and performance-optimized multicolor RUO/GMP panels are also available.
* as stated in the Quality System Regulations (QSR) [U.S.Food and Drug Administration Reference 21CFR 820]
Review available single color reagents here
IVDR compliant single color reagents
Custom multicolor panel solutions are also available to drive workflow efficiency. These reagents, defined by the customer, are manufactured using proprietary BD Horizon™ Chroma Dried Paneland are stable for 12–18 months at room temperature.
A user-defined assay across three instruments shows the performance of Universal Setup on the BD FACSLyric™ System.
Watch the video about simple assay transfer on the BD FACSLyric™ Cell Analyzer.
Functions in the BD FACSuite™ Application Assist in Supporting Your 21 CFR Part 11 Compliance and Electronic Record Integrity
Our flagship analyzer, BD FACSLyric™ Flow Cytometer, is upgradeable to a 12-color configuration. It is powered by the BD FACSuite™ Application used for acquisition and analysis that helps to support 21 CFR Part 11 with password protection, electronic signatures, automatic record keeping and audit trails. We provide operational qualification (OQ) by a BD field service engineer for features relevant to 21 CFR Part 11 for the BD FACSLyric™ Flow Cytometer and the BD FACSuite™ Application.
The BD FACSDuet™ Premium System allows traceability of samples and worklists through carrier barcoding and supports ISO 15189-accredited labs with compliance with complete workflow traceability.
Automated systems and software such as the BD FACSuite™ Application include built-in functional controls that helps with the functional aspects of 21 CFR Part 11. However, there are limitations to what the tools can provide.
Global Install Base with Guaranteed Technical Service Support
With BD as your single partner for flow cytometry reagents, instruments and services you will benefit from synergies in user workflows and support provided. BD Technical Services provide installation qualification (IQ), operational qualification (OQ) and technical service support to minimize instrument downtime. Explore our contract manufacturing offering of multicolor panels in liquid, dried or lyophilized formats to minimize errors and time associated with manual cocktailing of reagents.
The BD FACSLyric™ Flow Cytometer has an install base of more than 3,000 units around the world. BD Biosciences has service engineers and application specialists available across North and South America, Africa, Europe and Asia to support your needs as you look to scale up operations.
All user-defined flow cytometry assays used for manufacturing and QC release of product must be validated and verified by the user. The user must comply with applicable local regulations and obtain appropriate health authority approval as necessary for their intended use.
The manufacturing of cell therapy products requires testing at multiple stages and thus requires a significant amount of time and effort to ensure that the instrumentation and assays deliver accurate results. This drives interest in methods to improve efficiency of these workflows.
The BD FACSLyric™ Flow Cytometer enables efficiency and productivity with:
- Distinctive assay portability feature that allows easy and efficient transfer of assays across sites
- Automated analysis using the BD ElastiGate™ Autogating Algorithm in the BD FACSuite™ Application v1.6, which enables 1.8X faster analysis
- Fluorescence compensation required only every 60 days
- Flexibility for acquisition using a universal loader with 21 different loading options including multiwell plates
- Automated laser alignment
In addition, flow cytometry sample preparation can be automated using the BD FACSLyric™ Flow Cytometer integrated with the BD FACSDuet™ Sample Preparation System.
Benefits of this solution include:
- Physical integration of the sample preparation system with the flow cytometer to provide a complete end-to-end walkaway solution
- On-board, automated antibody cocktail preparation, washing, centrifugation and sample transfer that eliminates risk of errors due to manual pipetting
- Support for different types of blood collection tubes and a wide variety of reagent vials
Download the BD FACSDuet™ Premium Sample Preparation System brochure.
See below for data from a clinical lab demonstrating the reduction in hands-on time gained when using a BD FACSDuet™ System to automate a standard wash assay.
Reduce Operator Hands-on-time with the BD FACSDuet™ Premium System

Hands-on time for manual, BD FACS™ Lyse Wash Assistant and BD FACSDuet™ Premium System three samples, 15-tube workflows
Study conducted at a U.S. clinical lab. Three workflows were compared for total processing time and hands-on time for manual, BD FACS™ Lyse Wash Assistant and BD FACSDuet™ Premium Sample Preparation System workflows. This process uses pre-cocktailed reagents and begins with pulling reagents out of the fridge for manual/BD FACS™ Lyse Wash Assistant workflows or loading specimens onto the BD FACSDuet™ Premium System. Three specimens were run with an SLW assay with five tubes per specimen for a total of 15 tubes per workflow.
Consistent Reagent and Instrument Performance and Streamlined AssayTransfer Enables Global Standardization
Ensuring consistent performance across the globe is critical for the distributed manufacturing and testing commonly found in cell therapy manufacturing as well as producing quality clinical research data. BD Biosciences has developed robust methods to make this easier.
Streamlined Assay Transfer for Reproducible Performance Across Manufacturing Sites
Universal Setup on the BD FACSLyric™ Flow Cytometer allows transfer of user-defined assays from one instrument to another, supporting standardization of conditions for reproducibility across global manufacturing sites. This high-performance, highly sensitive flow cytometer demonstrates exceptional resolution and improved separation to make dim and rare populations easier to resolve.
The BD FACSDuet™ Sample Preparation System fully automates flow cytometry sample prep, including sample washing. The BD FACSDuet™ System adds traceability and automation to the preanalytical process.
BD Clinical Discovery Research Reagents are manufactured in an ISO 9001:2015 and ISO 13485:2016 certified manufacturing site to support your manufacturing QC assays. Using high quality reagents as part of your flow cytometry quality control helps to further standardize your manufacturing QC assay to give you confidence in the safety and integrity of your clinical research. High quality enables results that increase confidence in the data and that it can be safe for translating into clinical use in the future.
BD RUO (GMP) Reagents are manufactured in accordance with current Good Manufacturing Practices. Unit-sized preformulated and performance-optimized multicolor RUO/GMP panels are also available.
* as stated in the Quality System Regulations (QSR) [U.S.Food and Drug Administration Reference 21CFR 820]
Review available single color reagents here
IVDR compliant single color reagents
Custom multicolor panel solutions are also available to drive workflow efficiency. These reagents, defined by the customer, are manufactured using proprietary BD Horizon™ Chroma Dried Paneland are stable for 12–18 months at room temperature.
A user-defined assay across three instruments shows the performance of Universal Setup on the BD FACSLyric™ System.

Watch the video about simple assay transfer on the BD FACSLyric™ Cell Analyzer.
Functions in the BD FACSuite™ Application Assist in Supporting Your 21 CFR Part 11 Compliance and Electronic Record Integrity
Our flagship analyzer, BD FACSLyric™ Flow Cytometer, is upgradeable to a 12-color configuration. It is powered by the BD FACSuite™ Application used for acquisition and analysis that helps to support 21 CFR Part 11 with password protection, electronic signatures, automatic record keeping and audit trails. We provide operational qualification (OQ) by a BD field service engineer for features relevant to 21 CFR Part 11 for the BD FACSLyric™ Flow Cytometer and the BD FACSuite™ Application.
The BD FACSDuet™ Premium System allows traceability of samples and worklists through carrier barcoding and supports ISO 15189-accredited labs with compliance with complete workflow traceability.
Automated systems and software such as the BD FACSuite™ Application include built-in functional controls that helps with the functional aspects of 21 CFR Part 11. However, there are limitations to what the tools can provide.
Global Install Base with Guaranteed Technical Service Support
With BD as your single partner for flow cytometry reagents, instruments and services you will benefit from synergies in user workflows and support provided. BD Technical Services provide installation qualification (IQ), operational qualification (OQ) and technical service support to minimize instrument downtime. Explore our contract manufacturing offering of multicolor panels in liquid, dried or lyophilized formats to minimize errors and time associated with manual cocktailing of reagents.
The BD FACSLyric™ Flow Cytometer has an install base of more than 3,000 units around the world. BD Biosciences has service engineers and application specialists available across North and South America, Africa, Europe and Asia to support your needs as you look to scale up operations.

All user-defined flow cytometry assays used for manufacturing and QC release of product must be validated and verified by the user. The user must comply with applicable local regulations and obtain appropriate health authority approval as necessary for their intended use.
Advanced Flow Cytometry for Cell Therapy Manufacturing QC
BD was delighted to partner with CGT Catapult to support their multiple process analytical technologies (PAT) Consortium — a collaboration between leading companies to test novel PATs as applied to an exemplar 8-day CAR-T cell culture bioprocess. BD worked with CGT Catapult to develop a novel, 12-colour flow cytometry panel for the BD FACSLyric™ flow cytometer which was used as a product characterisation step for process QC. Read the white paper to learn more.
CGT Catapult Collaboration with BD on the PAT Consortium Project
The Cell and Gene Therapy (CGT) Catapult is an innovation and technology organisation committed to the advancement of cell and gene therapies. BD have partnered with CGT Catapult on the process analytical technologies (PAT) Consortium project, providing expertise in flow cytometry to support the consortium’s goal of making cell and gene therapies safer, more effective, scalable and affordable. Read CGT Catapult’s testimonial to discover more about the collaboration.
Cell and (CAR) T-Cell Therapy Research and Development
A common theme across CAR-T and other cell therapy research programs is the need to understand cell phenotypes and heterogeneity at every step of the development process. From the early steps in exploratory studies to the manufacturing QC process and clinical trials of cell therapies, BD Biosciences offers a comprehensive set of solutions that can help maximize your success. Learn more.
Clinical Trials: BD offers integrated solutions from sample preparation to data analysis to provide accurate, consistent results in your clinical development program. The need for standardised, certified techniques that can demonstrate compliance with regulations is essential to achieve consistency and efficiency, reduce error and inter-operator variability, and enable reproducibility over multiple centers. Learn more.
Companion Diagnostics (CDX): BD has an experienced team and leading flow cytometry technologies to support comprehensive CDx co-development from discovery to global commercialization. We offer the flexibility to collaborate at any stage of therapeutic, biomarker or diagnostic development. Learn more about our companion diagnostics partnership.
Submit your inquiry to have a BD representative reach out to you
*Required fields
-
White Papers
-
Application Notes
-
Brochures
-
Testimonials
-
Videos
BD FACSLyric™ Flow Cytometers and BD FACSDuet™ Sample Preparation System are Class 1 Laser Products.
BD FACSLyric™ Flow Cytometer is an open system for which CE-IVD applications have been validated by BD and the system can also be used for user defined assays/ protocols which are to be validated by the user. Refer to the IFU for further details.
![]()
BD FACSDuet™ Sample Preparation System and BD FACSLyric™ Flow Cytometer with the BD FACSuite™ Clinical and BD FACSuite™ Applications are in vitro diagnostic medical devices bearing a CE mark.
Custom panels are for are for Research Use Only. Not for use in diagnostic or therapeutic procedures.

