-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- BD OMICS-One™ WTA Next Assay
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?
Instrument Software
Explore BD Biosciences software offering that, paired with our instruments, supports your flow cytometry application set up, acquisition and analysis.
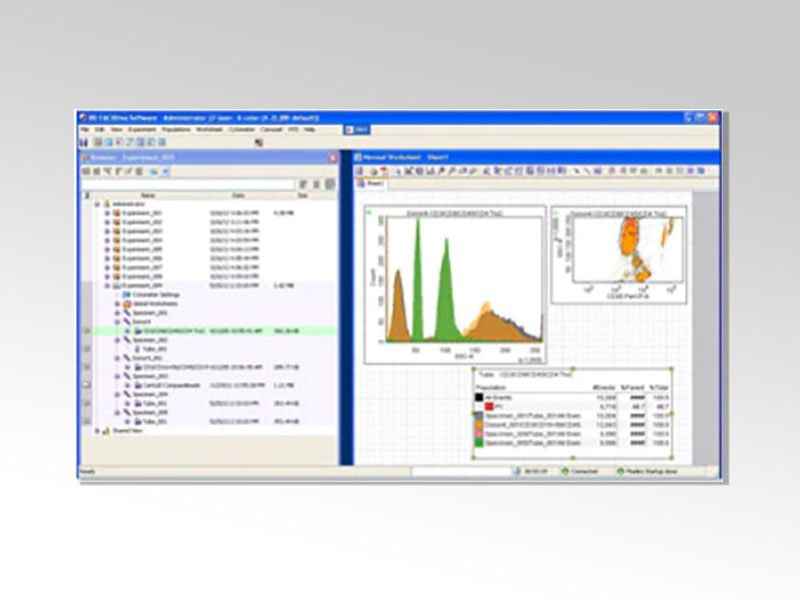
BD FACSDiva™ Software
BD FACSDiva™ Software is a collection of rich tools for flow cytometer application setup, data acquisition, and data analysis that help streamline flow cytometry workflows. The software also provides specialized features to help you integrate flow systems into application areas, such as index sorting for stem cell and single-cell applications, as well as automation protocols for high-throughput and robotic laboratories.
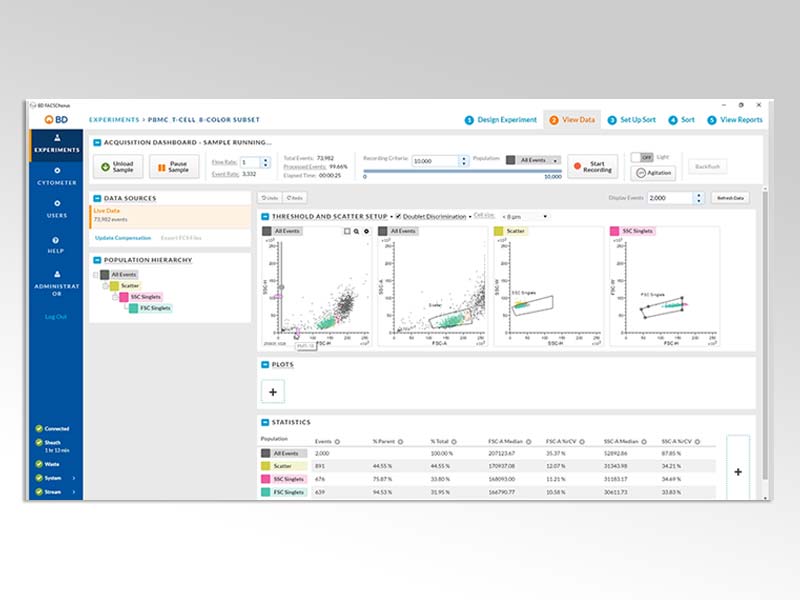
BD FACSChorus™ Software
BD FACSChorus™ Software simplifies sorting by using a streamlined workflow that eliminates manual setup and monitoring. The software guides you through the entire cell sorting process by using advanced automation technology that improves throughput and minimizes downtime.
BD FACSuite™ Clinical and BD FACSuite™ Applications
The software that controls the BD FACSLyric™ Flow Cytometry System is comprised of two applications, BD FACSuite™ Clinical Application and BD FACSuite™ Application. Both applications provide an easy-to-use interface for data acquisition and analysis with the BD FACSLyric™ System and features with many functions and options relevant to 21 CFR Part 11.

BD FACSCanto™ Clinical Software
BD FACSCanto™ Clinical Software is for use with the BD FACSCanto™ Flow Cytometer and includes features such as automated setup and compensation using BD FACS™ 7-Color Setup Beads, application-specific analysis modules and predefined reports with integrated cytometer quality control.

BD flow cytometers are Class 1 Laser Products.
The BD FACSCanto™ Flow Cytometer is for In Vitro Diagnostic Use. CE marked in compliance with the European In Vitro Diagnostic Medical Device Directive 98/79/EC
The BD FACSLyric™ flow cytometer with the BD FACSuite™ Clinical and BD FACSuite™ applications are CE marked in compliance with the European In Vitro Diagnostic Medical Device Directive 98/79/EC.
BD FACSChorus™ Software is for Research Use Only. Not for use in diagnostic or therapeutic procedures.
BD FACSDiva™ Software is for In Vitro Diagnostic Use. CE marked in compliance with the European In Vitro Diagnostic Medical Device Directive 98/79/EC.