-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- BD OMICS-One™ WTA Next Assay
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?

HIV Research
Human immunodeficiency virus (HIV) targets the immune system, and if untreated, causes acquired immune deficiency syndrome (AIDS). Around the world, 38 million individuals are currently living with HIV. Of those, 1.7 million people were newly infected in 2019 and 690,000 have died of AIDS-related illnesses.1 Two main types of HIV have been described—HIV-1 and HIV-2. They are very similar structurally and they both ultimately lead to AIDS. HIV-1 is more widely spread while HIV-2 is more restricted to Western and Central Africa. HIV-2 is less virulent with a longer progression into AIDS but induces diseases of the central nervous system more frequently.
What are the stages of HIV infection?
Assuming no treatment is taken, there are three phases in the progression of HIV infection:2
- Acute — usually occurs about 3 weeks post-exposure. During this phase, the virus replicates rapidly in CD4 T-cells, which is indicated by high viral load and high rate of CD4 T-cell destruction. This is when the host is the most infectious.
- Chronic — also known as clinical latency or asymptomatic HIV infection, the chronic phase is the second stage of infection. Viral replication is low and without treatment, those infected will progress to AIDS within 10+ years.
- AIDS — at this point, the virus has tremendously damaged the immune system and the host can no longer fight opportunistic infections.
How is HIV diagnosed?
Antibody tests are the most widely used diagnostic tests to detect HIV infection. It can take about 28 days post-exposure (window period) for HIV-specific antibodies to be detected. Nucleic acid tests (NAT) help determine the viral load in patients.
Biology of HIV infection
HIV is a retrovirus with a genome of 9.8 kilobases coding for a very small number of proteins and with a high mutation rate. The lipid envelope surrounding the core is derived from host cells and is studded with glycoproteins, which are of paramount importance during infection and for eliciting immunogenicity. The envelope protein gp120 binds to CD4 to fuse with T-cells and macrophages. Upon entry into the host cell, the viral RNA is reverse transcribed to DNA, which then integrates into the host genome and gets replicated using the hijacked host machinery. This results in the activation of immune responses instantly, resulting ultimately in depletion of the CD4+ T-cell population through various mechanisms.2
HIV-mediated CD4+ T-cell depletion
HIV-mediated CD4+ T-cell depletion is believed to occur through several stages—(i) an enhanced T-cell production upon infection, (ii) accelerated destruction of T-cells through immune responses, (iii) an accelerated T-cell production as a response to T-cell depletion through cytokine signaling in the lymph nodes, (iv) accelerated viral replication resulting in the destruction of progenitor cells in the bone marrow, thymus and the peripheral lymphoid systems.3
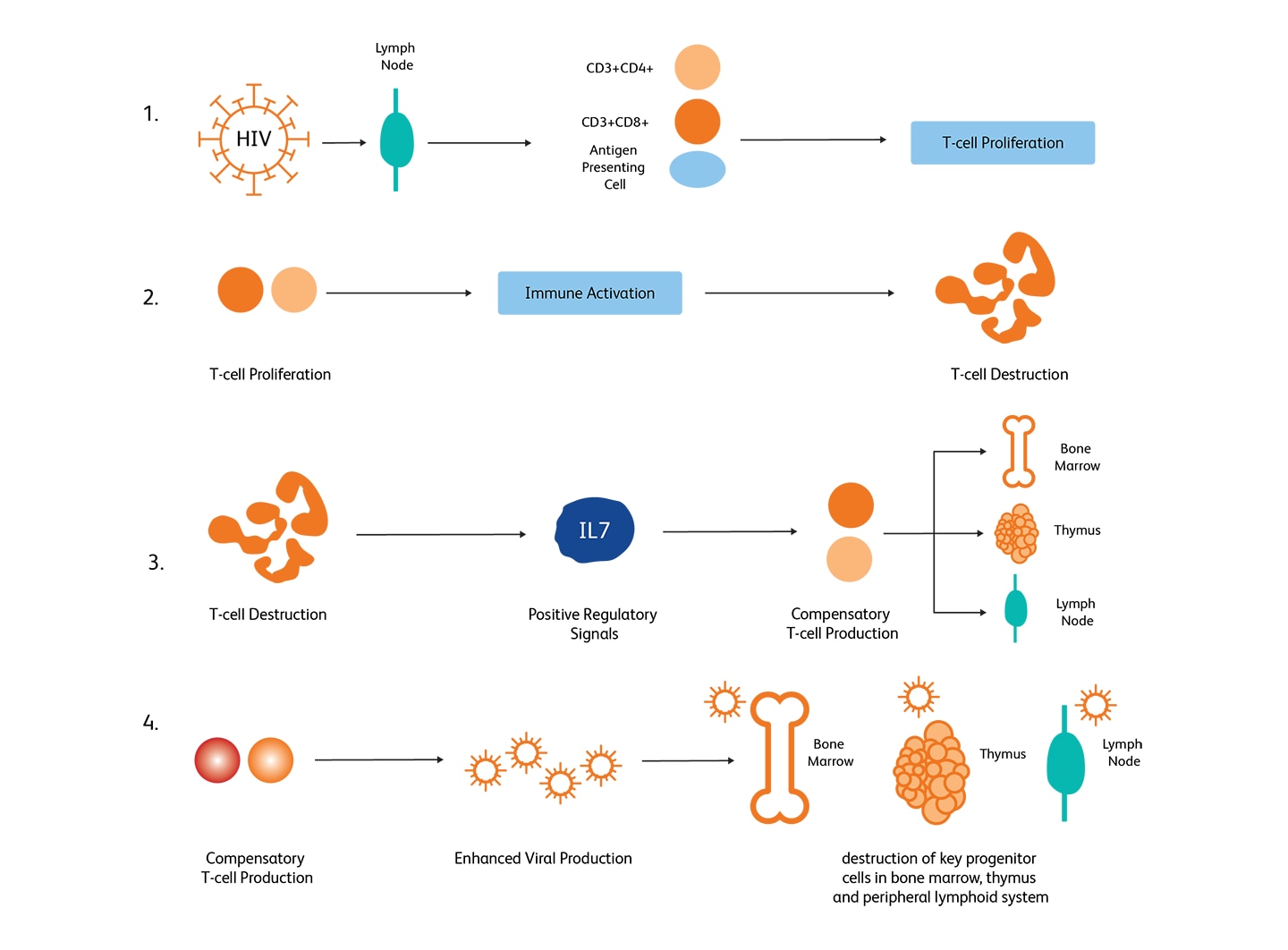
Mechanisms of HIV-mediated CD4+ T-cell depletion. (Image adapted from McCune, 2001).6
How is HIV monitored?
Viral load monitoring and CD4 T-cell count are routinely used for HIV monitoring. Per WHO and US CDC guidelines, CD4 T-cell count measurements are critical for gaining insights into HIV disease progression, for making clinical considerations on implementing antiviral therapy (ART), and for monitoring responses to ART.4,5
Determination of percent and absolute number of CD4+ T-lymphocytes using flow cytometry
Flow cytometry is a standard technique used for determining CD4+ T-lymphocytes counts as it provides accuracy, precision and reproducibility. Flow cytometry also provides high-throughput capabilities. Both percentage CD4+ T-lymphocytes among lymphocytes and absolute lymphocyte counts can be determined using flow cytometry. Dual-platform approaches for determining CD4 counts use a flow cytometer for generating percentage CD4+ T-lymphocytes and a hematological analyzer for enumerating absolute lymphocyte counts. Single-platform approaches determine CD4 counts without using a hematological analyzer by either directly counting the number of CD4+ T-lymphocytes in a given volume of blood by CD45 gating, using software with automated gating capabilities and beads.6 Determining percentages or cell counts of CD3+CD4+ lymphocytes can be useful for HIV-infected individuals.6 Individuals with HIV typically exhibit a steady decrease of CD3+CD4+ lymphocyte counts as the infection progresses.
Using adoptive transfer of costimulated CD4+ T-cells for reconstituting CD4+ helper T-cell activity has been demonstrated to be a possible method to augment natural immunity to HIV-1 infection.7 HIV-mediated immunosuppression could also result in patients becoming more prone to frequent and severe infections. At the advanced stages of infection, CD4 counts go below 200 CD4/µl,2 resulting in severe immunodeficiency.8 Opportunistic infections leading to diseases such as Pneumocystis pneumonia (PCP), toxoplasma encephalitis, cytomegalovirus (CMV) infections and tuberculosis are hallmarks of AIDS.9
Basic and clinical research on HIV have unraveled several critical aspects of HIV infection and transmission, such as the basic biology of HIV, identification of CD4 as the main HIV receptor, understanding HIV restriction factors, such as tetherin and tripartite motif-containing 5α (TRIM5α), and identifying microbial translocation as the pathogenic process.10 Research and clinical trials to develop an HIV vaccine are also underway.11 Translational research on biomedical prevention has also been progressing steadily.
Besides offering IVD assays for immune assessment (e.g., CD4 count) of HIV patients, BD Biosciences offers a variety of flow cytometers and flow cytometry research reagents that can meet different needs of HIV researchers. The dried, unit-sized, preformulated and optimized BD® Small Batch Multicolor Panels further offer a solution to streamline experimental workflow and standardize flow cytometry assays when performing large-scale or longitudinal studies.
References
- World Health Organization. HIV/AIDS Fact Sheet. https://www.who.int/news-room/fact-sheets/detail/hiv-aids. Published July 6, 2020. Accessed September 30, 2020
- Cowley S. The biology of HIV infection. Lepr Rev. 2001;72(2):212-220. doi: 10.5935/0305-7518.20010028
- McCune JM. The dynamics of CD4+ T-cell depletion in HIV disease. Nature. 2001;410(6831):974-979. doi: 10.1038/35073648
- World Health Organization. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. https://apps.who.int/iris/bitstream/handle/10665/255884/9789241550062-eng.pdf;jsessionid=8FA763DD24F996D16BF1C6FDCAFD8BA0. Published July 2017. Accessed November 12, 2020
- Centers for Disease Control and Prevention. HIV surveillance. Revised surveillance case definition for HIV infection – United Sates, 2014. https://www.cdc.gov/hiv/guidelines/reporting.html. Accessed November 12, 2020
- Center for Disease Control and Prevention. Guidelines for performing single-platform absolute CD4+ T-cell determinations with CD45 gating for persons infected with human immunodeficiency virus. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5202a1.htm. Published January 31, 2003. Accessed October 4, 2020.
- Levine BL, Bernstein WB, Aronson NE, et al. Adoptive transfer of costimulated CD4(+) T cells induces expansion of peripheral T cells and decreased CCR5 expression in HIV infection. Nat Med. 2002;8(1):47-53. doi: 10.1038/nm0102-47
- Streicher HZ, Reitz MS Jr, Gallo RC. Human immunodeficiency viruses. In Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. New York: Churchill Livingstone; 2000.
- National Institutes of Health. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/Adult_OI.pdf
- Barre-Sinoussi F, Ross AL and Delfraissy J-F. Past, present and future: 30 years of HIV research. Nat Rev Microbiol. 2013;11(12):877-883. doi: 10.1038/nrmicro3132
- Hsu DC and O’Connell RJ. Progress in HIV vaccine development. Hum Vaccin Immuother. 2017;13(5):1018-1030. doi: 10.1080/21645515.2016.1276138.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.