-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- Italy (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
BD Pharmingen™ Recombinant Human IP-10


Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Product Details
Description
Interferon-γ inducible protein-10 (IP-10) is a 10 kD protein that belongs to CXC chemokine family. Unlike other CXC chemokines, IP-10 is a chemoattractant for activated lymphocytes, but not resting lymphocytes or neutrophils. The gene for human IP-10 has been mapped to chromosome 4q2. CXC chemokine receptor 3 (CXCR3) has been identified as the receptor for IP-10.
Purity: The recombinant human IP-10 was produced and found to be ≥ 95% pure by SDS-PAGE analysis and visualized by silver strain.
Preparation And Storage
Recombinant human IP-10 is supplied lyophilized from a solution of aqueous buffered solution containing 50 µg BSA per 1 µg of cytokine as carrier protein. No sodium azide has been added to the preparation and the endotoxin level is less than 1 EU per 1 µg of the cytokine as determined by the LAL method. The product can be reconstituted in sterile neutral buffer containing not less than 0.5 - 10.0 mg/ml carrier protein such as human or bovine serum albumin, aliquoted into polypropylene microtubes and stored at -80°C. NOTE: Failure to add carrier protein** or store at indicated temperatures may result in a loss of activity.
Recommended Assay Procedures
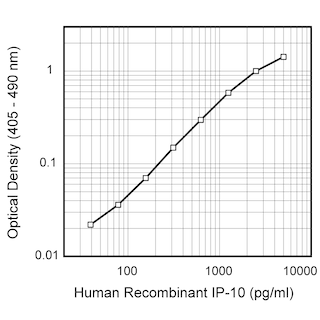
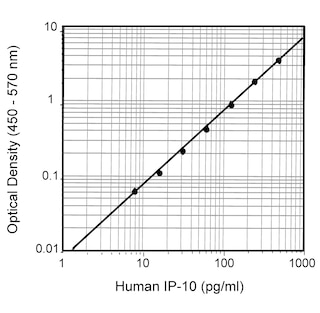
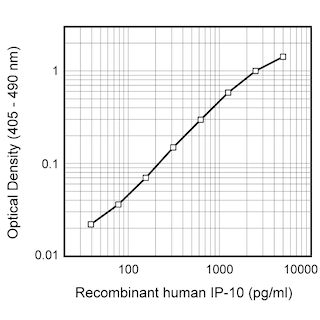
ELISA Standard: The recombinant human IP-10 (Cat. No. 551130) is useful as a quantitative standard for a sandwich ELISA for measuring human IP-10 protein levels. Purified 4D5 antibody (Cat. No. 555046) can be paired with the biotinylated 6D4 antibody (Cat. No. 555048) with recombinant human IP-10 as the standard. To obtain linear standard curves, doubling dilutions of human IP-10 ranging from ~1,000 to 5 pg/ml are recommended for inclusion in each ELISA plate. For specific methodology please visit the protocols sections or the chapter on ELISA in the Immune Function Handbook, both of which are posted on our web site, www.bdbiosciences.com.
Note: This ELISA pair is recommended primarily for measuring cytokine from experimental cell culture systems. These ELISA reagents are not recommended for assaying serum or plasma samples. For measuring human-IP-10 in serum or plasma our BD OptEIA™ Human IP-10 Set (Cat. No. 550926) is specially formulated and recommended.
Note: Biological Activity: This preparation of recombinant human IP-10 protein has not been evaluated for biological activity.
**Carrier proteins should be pre-screened for possible effects in an appropriate experimental system. Carrier proteins may effect experimental results due to toxicity, high endotoxin levels or possible blocking activity.
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
Data Sheets
Companion Products
Development References (4)
-
Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol. 1997; 61(3):246-257. (Biology). View Reference
-
Loetscher M, Gerber B, Loetscher P, et al. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996; 184(3):963-969. (Biology). View Reference
-
Luster AD, Jhanwar SC, Chaganti RS, Kersey JH, Ravetch JV. Interferon-inducible gene maps to a chromosomal band associated with a (4;11) translocation in acute leukemia cells. Proc Natl Acad Sci U S A. 1987; 84(9):2868-2871. (Biology). View Reference
-
Luster AD, Ravetch JV. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10). J Exp Med. 1987; 166(4):1084-1097. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.