Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD Pharmingen™ Biotin Annexin V
(RUO)



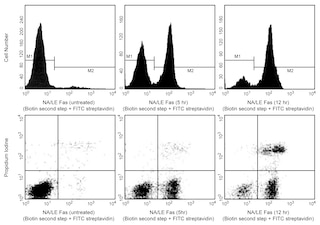
Biotin Annexin V: A tool for identifying cells that are undergoing apoptosis. Jurkat T cells were left untreated (upper left & lower left panels), treated for 5 hours (upper middle & lower middle panels) or 12 hours (upper right & lower right panels) with anti-human Fas antibody (clone DX2, Cat. No. 555670) and Protein G. Cells were incubated with Biotin Annexin V, followed by incubation with SAv-FITC in a buffer containing Propidium Iodide (PI). Cells were then analyzed by flow cytometry. Untreated cells were primarily Biotin Annexin V and PI negative, indicating that they were viable and not undergoing apoptosis. After a 5 hr treatment with DX2, there were two populations of cells: Cells undergoing apoptosis (Biotin Annexin V positive and PI negative), and cells that were viable and not undergoing apoptosis (Biotin Annexin V and PI negative). After a 12 hr treatment with DX2, three populations of cells were identified: Cells that had already died or were in late stage of apoptosis (Biotin Annexin V and PI positive), cells undergoing apoptosis (Biotin Annexin V positive and PI negative), and cells that were viable and not undergoing apoptosis (Biotin Annexin V and PI negative). The addition of Protein G enhances the ability of DX2 to induce apoptosis, presumably by cross-linking the Fas receptor.


BD Pharmingen™ Biotin Annexin V

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
Biotin Annexin V is a sensitive probe for identifying apoptotic cells, binding to negatively charged phospholipid surfaces (Kd of ~5 x 10e-2) with a higher affinity for phosphatidylserine (PS) than most other phospholipids. Biotin Annexin V binding is calcium dependent and defined calcium and salt concentrations are required for optimal staining as described in the Biotin Annexin V Staining Protocol. Investigators should note that Biotin Annexin V flow cytometric analysis on adherent cell types (e.g HeLa, NIH 3T3, etc.) is not routinely tested as specific membrane damage may occur during cell detachment or harvesting. Methods for utilizing Annexin V for flow cytometry on adherent cell types, however, have been previously reported (Casiola-Rosen et al. and van Engelend et al.).
INDUCTION OF APOPTOSIS USING AN ANTI-HUMAN CD95 (FAS) ANTIBODY
The following protocol is provided as an illustration on how Biotin Annexin V may be used on a human cell line.
Materials
1. A cell line or primary cells that can easily be induced to undergo apoptosis by human Fas mAb. Examples include Daudi lymphoma cells (ATCC CCL-213) and Jurkat T cells (ATCC TIB-152). It is important to note that there can be significant variation between cell lines regarding the level of apoptosis that can be induced through the Fas receptor. Also, not all cell types which express the Fas antigen will necessarily undergo Fas-mediated apoptosis. The cell lines mentioned above are good positive controls as they are strongly induced to undergo apoptosis by Fas mAb.
2. Anti-human CD95 (Fas) mAb, clone DX2 (Cat. No. 555670).
3. Recombinant Protein G (Sigma-Aldrich cat.no. P4689). We have found that the addition of Protein G to the tissue culture medium can significantly enhance the efficiency of the DX2 clone to induce apoptosis.
4. T25 tissue culture flasks.
5. IMDM or RPMI 1640 medium with 10% heat-inactivated fetal bovine serum (FBS), 1% L-glutamine and 1% antibiotics (penicillin/ streptomycin; 100 U/ml). This supplemented medium is simply referred to as 'medium' below.
Procedure
1. Maintain the cells in culture and change the medium one day before inducing apoptosis.
2. Induction of apoptosis: Add 0.5 - 2 µg/ml of the anti-CD95 antibody (DX2 clone) and 1-2 µg/ml Protein G to a T25 flask with medium containing ~0.5 × 10e6 cells/ml. Negative controls should consist of:
(a) ~0.5 × 10e6 cells/ml with medium alone (no mAb or Protein G), and
(b) ~0.5 × 10e6 cells/ml with medium and 1 µg/ml Protein G alone (no mAb).
3. Incubate the cells for 2 to 12 hr at 37°C
4. Proceed with the Biotin Annexin V Staining Protocol to measure apoptosis. Apoptosis can also be observed by light microscopy, gel electrophoresis (DNA fragmentation ladders) or by using a DNA fragmentation-based flow cytometry assay system such as the APO-BRDU™ Kit (Cat. No. 556405) or the APO-DIRECT™ Kit (Cat. No. 556381).
INDUCTION OF APOPTOSIS USING AN ANTI-MOUSE CD95 (FAS) ANTIBODY
The following protocol is provided as an illustration on how Biotin Annexin V may be used on murine cells.
Materials
1. A cell line or primary cells that can easily be induced to undergo apoptosis by mouse Fas monoclonal antibody (mAb). Thymocytes isolated from a 4-6 week old BALB/c mouse may be used.
2. Anti-mouse CD95 (Fas) mAb, clone Jo2 (Cat. No. 554254)
3. Recombinant Protein G (Sigma-Aldrich cat.no. P4689). We have found that the addition of Protein G to the tissue culture medium can significantly enhance the efficiency of Jo2 mAb to induce apoptosis.
4. T25 tissue culture flasks
5. RPMI-1640 medium with 10% heat-inactivated fetal bovine serum (FBS), 1% L-glutamine and 1% antibiotics (penicillin/ streptomycin; 100 U/ml). This supplemented medium is simply referred to as 'medium' below.
Procedure
1. Isolate BALB/c thymocytes from the thymus of a 4-6 week old mouse.
2. Induction of apoptosis. Add 2.5-10 µg/ml Jo2 (Cat. No. 554254) and 1-2 µg/ml Protein G to a T25 flask containing ~2 × 10e6 thymocytes/ml. Negative controls should consist of
(a) ~2 × 10e6 thymocytes/ml with medium alone (no mAb or Protein G)
(b) ~2 × 10e6 thymocytes/ml with medium and 1-2 µg/ml Protein G alone (no mAb).
3. Incubate the cells for 2-12 hrs at 37°C.
4. Proceed with the Biotin Annexin V Staining Protocol to measure apoptosis. Apoptosis can also be observed by light microscopy, gel electrophoresis (DNA fragmentation ladders) or by using a DNA fragmentation-based flow cytometry assay system such as the APO-BRDU™ Kit (Cat. No. 556405) or the APO-DIRECT™ Kit (Cat. No. 556381).
BIOTIN ANNEXIN V STAINING PROTOCOL
Biotin Annexin V is used to quantitatively determine the percentage of cells within a population that are actively undergoing apoptosis. It relies on the property of cells to lose membrane asymmetry in the early phases of apoptosis. In apoptotic cells, the membrane phospholipid phosphatidylserine (PS) is translocated from the inner leaflet of the plasma membrane to the outer leaflet, thereby exposing PS to the external environment. Annexin V is a calcium-dependent phospholipid-binding protein that has a high affinity for PS, and is useful for identifying apoptotic cells with exposed PS. Propidium Iodide (PI) is a standard flow cytometric viability probe and is used to distinguish viable from nonviable cells. Viable cells with intact membranes exclude PI, whereas the membranes of dead and damaged cells are permeable to PI. Cells that stain positive for Biotin Annexin V and negative for PI are undergoing apoptosis. Cells that stain positive for both Biotin Annexin V and PI are either in the end stage of apoptosis, are undergoing necrosis, or are already dead. Cells that stain negative for both Biotin Annexin V and PI are alive and not undergoing measurable apoptosis.
Reagents
1. Biotin Annexin V: Included. Use 5 µl per test.
2. Propidium Iodide (PI): Not Included. PI (cat.no. 556463) is a convenient, ready-to-use nucleic acid dye. Use up to 10 µl per test of a 50 µg/ml solution.
3. 10× Binding Buffer: Not Included. 0.1 M Hepes (pH 7.4), 1.4 M NaCl, 25 mM CaCl2. Store at 4°C. Alternatively, catalog number 556454 may be purchased.
4. FITC-Streptavidin (cat.no. 554060): Not Included.
Staining
1. Wash cells twice with cold PBS and then resuspend cells in 1× Binding Buffer at a concentration of 1 × 10e6 cells/ml.
2. Transfer 100 µl of the solution (1 × 10e5 cells) to a 5 ml culture tube.
3. Add 5 µl of Biotin Annexin V.
4. Gently vortex the cells and incubate for 15 min at RT (25 °C) in the dark.
5. Wash once with 1ml of 1× Binding Buffer.
6. Dilute 0.5 µg FITC-Streptavidin into 100 µl 1× Binding Buffer and add to the cell pellet. Gently mix the cells.
7. Add 10 µl PI and incubate for 15 min at RT (25 °C). The optimal concentration of PI may vary among cell lines where 10 µl of a 50 µg/ml stock is most likely the maximum to be required. Less may yield optimal results in some experimental systems.
8. Add 400 µl of 1× Binding Buffer to each tube. Analyze by flow cytometry within 1 hr.
SUGGESTED CONTROLS FOR SETTING UP FLOW CYTOMETRY
The following controls are used to set up compensation and quadrants:
1. Cells stained only with FITC-Streptavidin.
2. Cells stained with Biotin Annexin V + FITC-Streptavidin (no PI).
3. Cells stained with PI + FITC-Streptavidin (no Biotin Annexin V).
Other Staining Controls:
A cell line that can be easily induced to undergo apoptosis should be used to obtain positive control staining with Biotin Annexin V and/or Biotin Annexin V and PI. It is important to note that the basal level of apoptosis and necrosis varies considerably within a population. Thus, even in the absence of induced apoptosis, most cell populations will contain a minor percentage of cells that are positive for apoptosis (Biotin Annexin V positive, PI negative or Biotin Annexin V positive, PI positive).
The untreated population is used to define the basal level of apoptotic and dead cells. The percentage of cells that have been induced to undergo apoptosis is then determined by subtracting the percentage of apoptotic cells in the untreated population from percentage of apoptotic cells in the treated population. Since cell death is the eventual outcome of cells undergoing apoptosis, cells in the late stages of apoptosis will have a damaged membrane and stain positive for PI as well as for Biotin Annexin V. Thus the assay does not distinguish between cells that have already undergone an apoptotic cell death and those that have died as a result of necrotic pathway, because in either case the dead cells will stain with both Biotin Annexin V and PI.
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
Companion Products

.png?imwidth=320)
Apoptosis is a normal physiologic process which occurs during embryonic development as well as in maintenence of tissue homeostasis. The apoptotic program is characterized by certain morphologic features, including loss of plasma membrane asymmetry and attachment, condensation of the cytoplasm and nucleus, and internucleosomal cleavage of DNA. Loss of plasma membrane is one of the earliest features. In apoptotic cells, the membrane phospholipid phosphatidylserine (PS) is translocated from the inner to the outer leaflet of the plasma membrane, thereby exposing PS to the external cellular environment. Annexin V is a 35-36 kDa Ca2+ dependent phospholipid-binding protein that has a high affinity for PS, and binds to cells with exposed PS. Annexin V may be conjugated to biotin. This format retains its high affinity for PS and thus serves as a sensitive probe for flow cytometric analysis of cells that are undergoing apoptosis. Since externalization of PS occurs in the earlier stages of apoptosis, Biotin Annexin V staining can identify apoptosis at an earlier stage than assays based on nuclear changes such as DNA fragmentation.
Biotin Annexin V staining precedes the loss of membrane integrity which accompanies the latest stages of cell death resulting from either apoptotic or necrotic processes. Therefore, staining with Biotin Annexin V is typically used in conjunction with a vital dye such as propidium iodide (PI) or 7-Amino-Actinomycin (7-AAD) to allow the investigator to identify early apoptotic cells (PI negative, Biotin Annexin V positive). Viable cells with intact membranes exclude PI, whereas the membranes of dead and damaged cells are permeable to PI. For example, cells that are considered viable are both Biotin Annexin V and PI negative while cells that are in early apoptosis are Biotin Annexin V positive and PI negative, while cells that are in late apoptosis or already dead are both Biotin Annexin V and PI positive. This assay does not distinguish between cells that have undergone apoptotic death versus those that have died as a result of a necrotic pathway because in either case, the dead cells will stain with both Biotin Annexin V and PI. However, when apoptosis is measured over time, cells can be often tracked from Biotin Annexin V and PI negative (viable, or no measurable apoptosis), to Biotin Annexin V positive and PI negative (early apoptosis, membrane integrity is present) and finally to Biotin Annexin V and PI positive (end stage apoptosis and death). The movement of cells through these three stages suggests apoptosis. In contrast, a single observation indicating that cells are both Biotin Annexin V and PI positive, in of itself, reveals less information about the process by which the cells underwent their demise.
Biotin Annexin V is routinely tested by flow cytometric analysis. Other applications were tested at BD Biosciences Pharmingen during antibody development only or reported in the literature.
Development References (8)
-
Andree HA, Reutelingsperger CP, Hauptmann R, Hemker HC, Hermens WT, Willems GM. Binding of vascular anticoagulant alpha (VAC alpha) to planar phospholipid bilayers. J Biol Chem. 1990; 265(9):4923-4928. (Biology). View Reference
-
Casciola-Rosen L, Rosen A, Petri M, Schlissel M. Surface blebs on apoptotic cells are sites of enhanced procoagulant activity: implications for coagulation events and antigenic spread in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1996; 93(4):1624-1629. (Methodology: Apoptosis, Flow cytometry). View Reference
-
Homburg CH, de Haas M, von dem Borne AE, Verhoeven AJ, Reutelingsperger CP, Roos D. Human neutrophils lose their surface Fc gamma RIII and acquire Annexin V binding sites during apoptosis in vitro. Blood. 1995; 85(2):532-540. (Biology: Apoptosis). View Reference
-
Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994; 84(5):1415-1420. (Methodology: Apoptosis, Flow cytometry). View Reference
-
Martin SJ, Reutelingsperger CP, McGahon AJ, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995; 182(5):1545-1556. (Biology: Apoptosis). View Reference
-
Raynal P, Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994; 1197(1):63-93. (Biology). View Reference
-
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995; 184(1):39-51. (Methodology: Apoptosis, Flow cytometry). View Reference
-
van Engeland M, Ramaekers FC, Schutte B, Reutelingsperger CP. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry. 1996; 24(2):131-139. (Methodology: Apoptosis, Flow cytometry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.