-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- BD OMICS-One™ WTA Next Assay
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?
By Your Side Through IVDR
The European In Vitro Diagnostic Medical Device Regulation (IVDR) (EU) 2017/746 came into effect on 26 May 2017 and replaces the European In Vitro Diagnostic Medical Device Directive (IVDD) 98/79/EC. A global cross-functional team at BD Biosciences has been working to implement the new regulation in a seamless and compliant manner.
EU IVDR Amending Regulations (EU) 2022/112 and (EU) 2023/607 expands the scope of the grace period to certain devices and the abolishment of the sell-off provisions of IVDR. Consequently, devices placed on the market before the end of the transition period can be made further available on the market without a time restriction.
We are committed to providing IVDR compliant solutions to continue delivering safe and effective IVD medical devices to serve you and your patients. We received our first IVDR product certification back in December 2020 and the majority of BD's previous IVDD compliant devices for flow cytometry are now IVDR compliant. We are continuing our efforts to achieve this for current and new products in our clinical portfolio.
The BD Biosciences IVDR compliant reagent portfolio includes in vitro diagnostic solutions for*
- Flow cytometric immunophenotyping of haematological disorders.
- Monitoring human immunodeficiency virus (HIV)–infected individuals.
- Characterising and monitoring immunodeficiency and autoimmune diseases.
- Cell enumeration of blood products for transfusion and stem cell transplantation procedures.
The BD Biosciences IVDR portfolio will also include flow cytometers and automation solutions.
*After the IVDR date of application, the products indicated on this page will be made available as IVDR compliant devices and/or as IVDD compliant devices
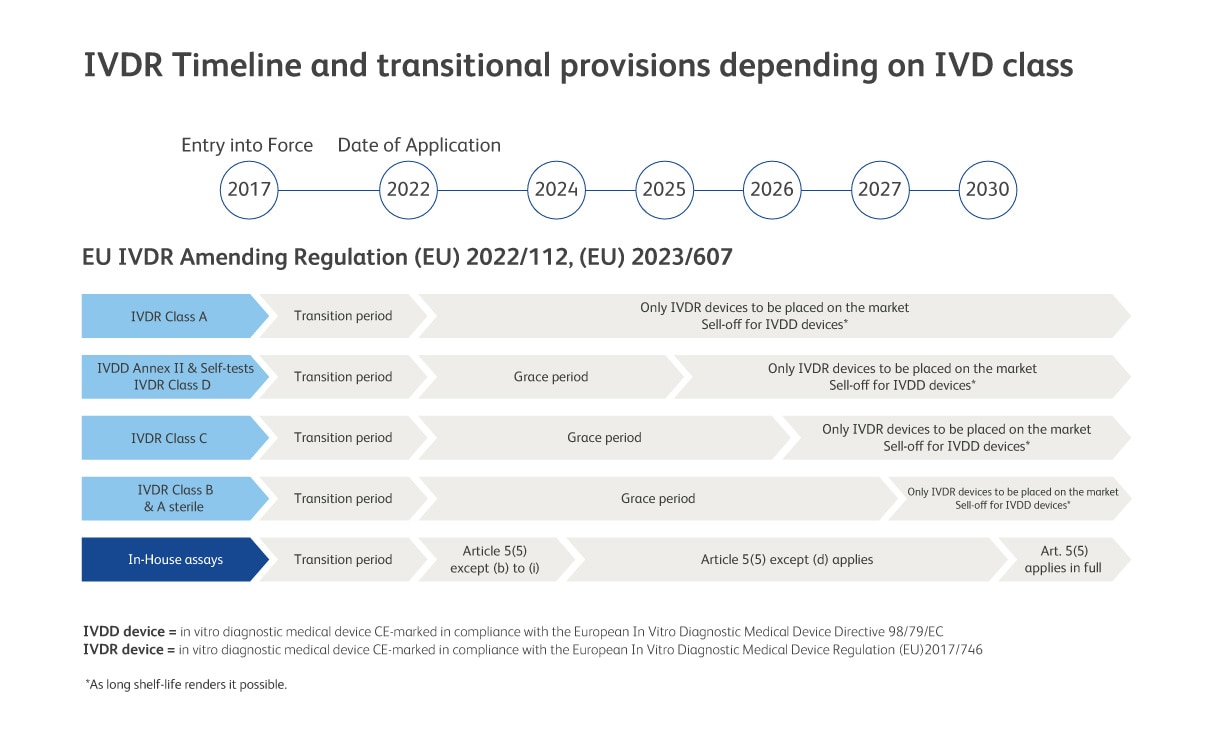
Read More about IVDR Article 5.5 and Upcoming Changes for LDT
Read More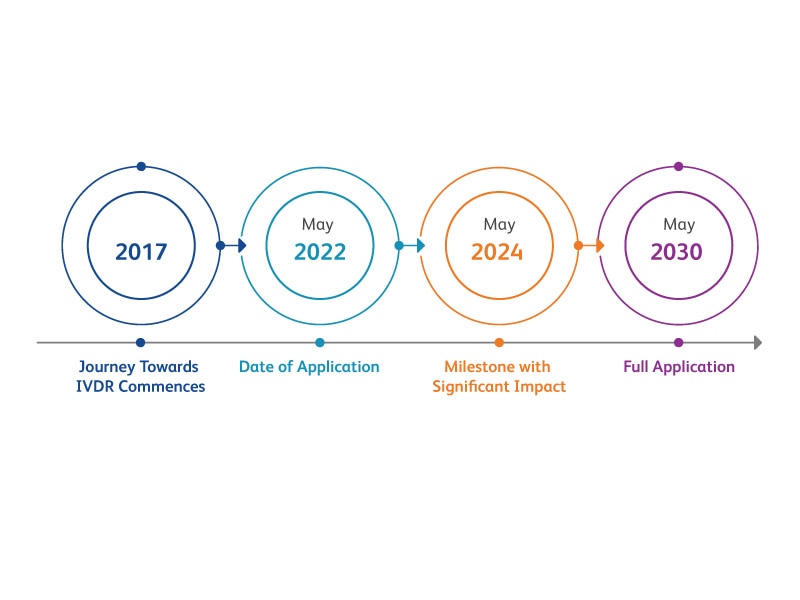
Key IVDR Article 5(5) milestones; impact to laboratories
The EU-IVDR regulatory framework not only impacts manufacturers of in vitro diagnostic medical devices (IVDs) but also, health institutions (HI) that use laboratory developed tests (LDTs). HIs, like labs*, can only use LDTs, if all the requirements of the entire IVD Regulation (EU) 2017/746 are met or the HI meets all the conditions of Article 5(5), in that case they are exempt from having to meet IVDR requirements in full and the LDT only needs to be compliant with the relevant general safety and performance requirements (GSPRs) in Annex I.
Furthermore, IVDR only allows the manufacture and use of an LDT if there is no equivalent CE-IVD device on the market or, if the HI demonstrates that a target patient-group’s specific needs cannot be met or cannot be met at the appropriate level of performance by an equivalent CE-IVD device.
The new IVDR sets high standards of quality and safety for in vitro diagnostic medical devices to ensure the highest level of public health protection.
*Labs within hospitals or laboratories and public health institutes that support the health care system and/ or address patient needs, but which do not treat or care for patients directly.
BD's IVDR Compliant Portfolio of Reagents
Haematological Malignancies
Leukemia and lymphoma and plasma cell disorders are complex multifactorial diseases and their diagnosis requires an interdisciplinary approach.
BD offers the BD OneFlow™ solution specifically designed for flow cytometry as an aid in the diagnosis of hematological malignancies. Built on the research and validation work of the EuroFlow™ Consortium for comprehensive immunophenotypic diagnosis and classification of haematological malignancies1, the BD OneFlow™ solution brings the standardisation of leukemia, lymphoma and plasma cell disorder immunophenotyping one step forward. It is made of a comprehensive set of reagents, protocols and assay templates to set up the flow cytometer, stain, acquire and analyse patient samples for immunophenotyping of normal and aberrant cell populations.
The BD OneFlow™ solution can improve laboratory efficiency and laboratories adopting it can expect safe and efficient processes.2,3
Lymphocyte Subsets
The BD Multitest™ Reagents are multicolor reagents that can be used with suitable flow cytometers to identify and determine the percentages and absolute counts of lymphocyte subsets in peripheral blood. BD Biosciences offers a choice of several reagent formats for lymphocyte subset testing and appropriate process controls. It also provides the option of absolute counts when paired with BD Trucount™ Tubes in a single-platform approach ensuring more accurate cell counts.
BD® Multi-Check Control and BD® Multi-Check CD4 Low Control are stable two-level controls with assigned values that can be used to monitor the immunophenotyping process.
Stem Cell Enumeration
The BD® Stem Cell Enumeration Kit with BD Trucount™ Tubes offers a single-tube, single-platform assay for accurate, reproducible, and rapid enumeration of CD34+ hematopoietic progenitor cells in a wide range of stem cell sources. An accurate measure of the CD34 cell count is necessary for dose requirement protocols in stem cell transplantation.
The BD® Stem Cell Control is intended as a complete, two-level process control with assigned values to monitor the immunophenotyping process for CD34+ cells.
Enumeration of Residual White Blood Cells (WBC) in Blood Products
Leucoreduction, or post-collection processing with special filters, can lower the white blood cell (WBC) count, minimizing complications associated with transfusions. BD Leucocount™ Kit, containing BD Leucocount™ Reagent and BD Trucount™ Tubes, is designed for accurate counting of residual WBCs in leucoreduced blood products. The BD Leucocount™ PLT and RBC controls are used to monitor methods for enumeration of residual leucocytes in leucoreduced platelet and RBC products respectively.
Single Colour Reagents
BD Biosciences offers a growing portfolio of antigen-fluorochrome combinations to choose from, for dim to very bright antigen expression. Our CE-IVD single-colour reagents are manufactured in an ISO 13485:2016 certified facility. BD is proud to be the only provider of CE-IVD brilliant violet reagents.
Review the BD single-colour reagent portfolio here.

BD FACSLyric™ Flow Cytometry
The BD FACSLyric™ flow cytometry solution combines simplicity, speed and automation to ease workflow and improve productivity. This next-generation flow cytometer enables flow cytometry workflow standardisation and collaboration through automation and unique assay portability capabilities.
The entire system, including the BD FACSuite™ Application and the BD FACSuite™ Clinical Applications, is now IVDR compliant. It also offers assay modules for BD CE‑IVD applications enabling automated gating for more standardised, efficient and less error-prone acquisition and analysis workflow.
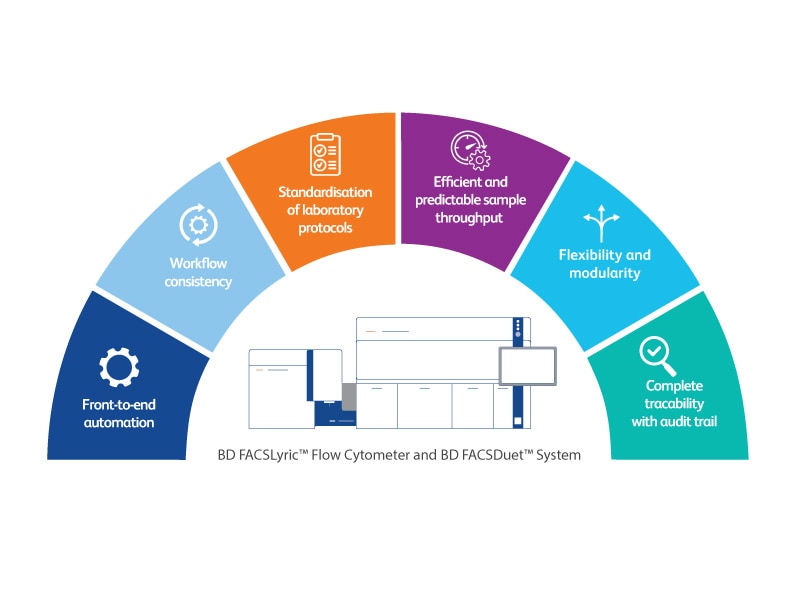
The BD FACSDuet™ System*
The BD FACSDuet™ System (BD FACSDuet™ Sample Preparation System and BD FACSDuet™ Premium Sample Preparation System) is designed to move the pace of your lab forward. With pre-analytical automation, process standardisation and automated cocktailing, the BD FACSDuet™ System drives efficiency and consistency in workflows. When physically integrated with the BD FACSLyric™ Flow Cytometer, the BD FACSDuet™ System delivers front-to-end automation with walkaway capabilities.
With on-board washing and on-board centrifugation, the BD FACSDuet™ Premium Sample Preparation System enhances lab efficiency, flexibility and standardisation of protocols, further reducing hands-on time, empowering labs to meet increasing workload demands.
* BD FACSDuet™ Sample Preparation System and BD FACSDuet™ Premium Sample Preparation System.

The BD FACS™ Lyse Wash Assistant
The BD FACS™ Lyse Wash Assistant automates sample preparation for flow cytometry applications. Equipped with patented cell wash technology, it lyses, mixes, washes and fixes samples with minimal manual intervention.
It enables automated batch processing of up to 40 sample tubes per run.

The BD FACSDuet™ System and BD FACSLyric™ Flow Cytometer
The physical integration of the BD FACSDuet™ System and the BD FACSLyric™ Flow Cytometer results in a front-to-end, walkaway, sample to answer solution. When not physically integrated, the data integration allows for the transfer of worklists from one or more BD FACSDuet™ Systems to one or more BD FACSLyric™ Flow Cytometers, increasing processing capabilities and flexibility of operations.
BD has an extensive CE-IVD compliant portfolio to support flow cytometry within oncohaematology, immunology, transfusion and transplantation laboratories with CE-IVD solutions and an expanding portfolio of single colour CE-IVD reagents. We offer a complete integrated clinical flow cytometry solution that simplifies and automates processes, with traceability and electronic audit trails, helping to drive compliance, automation, standardisation and quality. The BD clinical solution can help to keep moving forward on your journey to IVDR.
![]()
BD FACSLyric™ Flow Cytometer with the BD FACSuite™ Clinical and BD FACSuite™ applications, BD FACSDuet™ Sample Preparation System, BD FACSDuet™ Premium Sample Preparation System, BD Multitest™ Reagents (333184, 333185 only) and BD FACS™ Lyse Wash Assistant are in vitro diagnostic medical devices bearing a CE mark.
![]()
BD OneFlow™ PCST, BD OneFlow™ PCD, BD OneFlow™ LST, BD OneFlow™ B-CLPD T1, BD OneFlow™ ALOT, BD Multitest™ Reagents (except 333184, 333185), BD® Multi-Check Control, BD® Multi-Check CD4 Low Control, BD® Stem Cell Enumeration Kit, BD® Stem Cell Control, BD Trucount™ Tubes, BD Leucocount™ Kit , BD Leucocount™ Control (RBC, PLT and Combo) and the BD Single-Color Reagents (linked document) are in vitro diagnostic medical devices bearing a CE mark and are CE certified by BSI Group The Netherlands B.V. (Notified Body Number = 2797).
After the IVDR date of application, the products indicated on this page will be made available as IVDR compliant devices and/or as IVDD compliant devices.
BD Flow Cytometers, BD FACSDuet™ Premium Sample Preparation System and BD FACSDuet™ Sample Preparation System are Class 1 Laser Products.
- van Dongen JJM, Lhermitte L, Böttcher S, et al. on behalf of the EuroFlow Consortium (EU-FP6, LSHB-CT-2006-018708). EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26(9): 1908-1975. doi: 10.1038/leu.2012.120
- van der Velden VHJ, Flores-Montero J, Perez-Andres M, et al. Optimization and testing of dried antibody tube: The EuroFlow LST and PIDOT tubes as examples. J Immunol Methods. 2019;475:112287. doi: 10.1016/j.jim.2017.03.011
- Moloney E, Watson H, Barge D, et al. Efficiency and health economic evaluations of BD OneFlow™ Flow Cytometry Reagents for diagnosing chronic lymphoid leukemia. Cytometry B Clin Cytom. 2019;96(6):514-520. doi: 10.1002/cyto.b.21779
EuroFlow or any of the EuroFlow participants cannot be held liable for any claims or damages in connection with their services in the field of flow cytometry.

