Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?




Multicolor flow cytometric analysis of Ki-67 expression by proliferating Molt-4 and noncycling human peripheral blood mononuclear cells (PBMC). Proliferating Molt-4 cells and noncycling PBMC were fixed and permeabilized with 70% ice cold ethanol, washed, and stained with BD Horizon™ BV421 Mouse Anti-Ki-67 antibody (Cat. No. 562899/565929) according to the BD Biosciences support protocol, \"Flow Cytometry Staining Protocol for Detection of Ki-67.\" The cells were then counterstained with BD Via-Probe™ [Cat. No. 555815; contains 7-Amino-Actinomycin D (7-AAD)] to stain DNA. Two-color flow cytometric dot plots showing the correlated expression patterns of 7-AAD staining versus Ki-67 were derived from gated events with the forward and side light-scatter characteristics of intact Molt-4 cells (Left Panel) or PBMC (Right Panel). Flow cytometry was performed using a BD LSR™ II Flow Cytometer System.


BD Horizon™ BV421 Mouse Anti-Ki-67

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
For more information on detecting Ki-67 by flow cytometry please visit http://www.bdbiosciences.com/resources/protocols/detection_ki_67.jsp
BD™ CompBeads can be used as surrogates to assess fluorescence spillover (Compensation). When fluorochrome conjugated antibodies are bound to CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cells and CompBead to ensure that BD Comp beads are appropriate for your specific cellular application.
For optimal and reproducible results, BD Horizon Brilliant Stain Buffer should be used anytime two or more BD Horizon Brilliant dyes are used in the same experiment. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. The BD Horizon Brilliant Stain Buffer was designed to minimize these interactions. More information can be found in the Technical Data Sheet of the BD Horizon Brilliant Stain Buffer (Cat. No. 563794/566349) or the BD Horizon Brilliant Stain Buffer Plus (Cat. No. 566385).
Product Notices
- This reagent has been pre-diluted for use at the recommended Volume per Test. We typically use 1 × 10^6 cells in a 100-µl experimental sample (a test).
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Species cross-reactivity detected in product development may not have been confirmed on every format and/or application.
- Pacific Blue™ is a trademark of Molecular Probes, Inc., Eugene, OR.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- BD Horizon Brilliant Violet 421 is covered by one or more of the following US patents: 8,158,444; 8,362,193; 8,575,303; 8,354,239.
- BD Horizon Brilliant Stain Buffer is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,575,303; 8,354,239.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products


The B56 monoclonal antibody specifically binds to the Ki-67 antigen that is expressed in the nucleus of cycling cells (G1, S, G2, M cell cycle phases). During the G0 phase, the antigen cannot be detected. During interphase of the cell cycle, it is associated with nucleolar components, and it is on the surface of the chromosomes during M phase. Ki-67 is a large protein having 2 alternatively spliced isoforms, an N-terminal forkhead-associated domain, a C-terminal domain that binds to heterochromatin proteins, and multiple phosphorylation sites, the functions of which are still unclear. Because of the strict association of Ki-67 expression with cell proliferation, anti-Ki-67 antibodies are useful for the identification, quantification, and monitoring of growing cell populations.
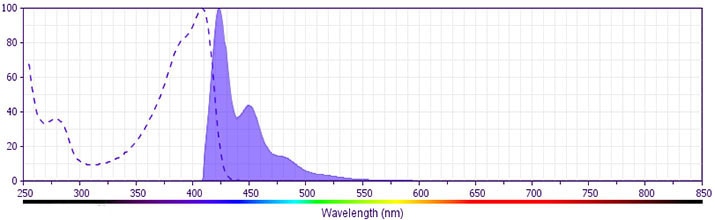
The antibody was conjugated to BD Horizon BV421 which is part of the BD Horizon Brilliant™ Violet family of dyes. With an Ex Max near 407 nm and Em Max near 421 nm, BD Horizon BV421 can be excited by the violet laser (405 nm) and detected with a 450/50 nm filter. BD Horizon BV421 conjugates are very bright, often exhibiting a 10 fold improvement in brightness compared to Pacific BlueTM conjugates. Due to nearly identical excitation and emission properties but different spillover characteristics, BD Horizon BV421, Pacific Blue, and BD Horizon V450 cannot be used simultaneously.
Development References (17)
-
Benson MJ, Elgueta R, Schpero W, et al. Distinction of the memory B cell response to cognate antigen versus bystander inflammatory signals. J Exp Med. 2009; 206(9):2013-2025. (Clone-specific: Flow cytometry). View Reference
-
Bigley V, Haniffa M, Doulatov S, et al. The human syndrome of dendritic cell, monocyte, B and NK lymphoid deficiency. J Exp Med. 2011; 208(2):227-234. (Clone-specific: Flow cytometry). View Reference
-
Bruno S, Crissman HA, Bauer KD, Darzynkiewicz Z. Changes in cell nuclei during S phase: progressive chromatin condensation and altered expression of the proliferation-associated nuclear proteins Ki-67, cyclin (PCNA), p105, and p34. Exp Cell Res. 1991; 196(1):99-106. (Biology: Flow cytometry). View Reference
-
Bruno S, Darzynkiewicz Z. Cell cycle dependent expression and stability of the nuclear protein detected by Ki-67 antibody in HL-60 cells. Cell Prolif. 1992; 25(1):31-40. (Biology: Flow cytometry). View Reference
-
Byeon IJ, Li H, Song H, Gronenborn AM, Tsai MD. Sequential phosphorylation and multisite interactions characterize specific target recognition by the FHA domain of Ki67.. Nat Struct Mol Biol. 2005; 12(11):987-93. (Biology). View Reference
-
Ho DWY, Fan ST, To J, et al. Selective plasma filtration for treatment of fulminant hepatic failure induced by D-galactosamine in a pig model. Gut. 2002; 50:869-876. (Clone-specific).
-
Kill IR. Localisation of the Ki-67 antigen within the nucleolus: evidence for a fibrillarin-deficient region of the dense fibrillar component. J Cell Sci. 1996; 109(6):1253-1263. (Biology). View Reference
-
Kouro T, Medina KL, Oritani K, Kincade PW. Characteristics of early murine B-lymphocyte precursors and their direct sensitivity to negative regulators. Blood. 2001; 97(9):2708-2715. (Clone-specific: Flow cytometry). View Reference
-
Kubbutat MH, Key G, Duchrow M, Schluter C, Flad HD, Gerdes J. Epitope analysis of antibodies recognising the cell proliferation associated nuclear antigen previously defined by the antibody Ki-67 (Ki-67 protein). J Clin Pathol. 1994; 47(6):524-528. (Biology). View Reference
-
Picker LJ, Hagen SI, Lum R, et al. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med. 2004; 200(10):1299-1314. (Clone-specific: Flow cytometry). View Reference
-
Pitcher CJ, Hagen SI, Walker JM, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002; 168(1):29-43. (Clone-specific: Flow cytometry). View Reference
-
Scholzen T, Endl E, Wohlenberg C, et al. The Ki-67 protein interacts with members of the heterochromatin protein 1 (HP1) family: a potential role in the regulation of higher-order chromatin structure.. J Pathol. 2002; 196(2):135-44. (Biology). View Reference
-
Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown.. J Cell Physiol. 2000; 182(3):311-22. (Biology). View Reference
-
Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991; 39(6):741-748. (Biology). View Reference
-
Spargo LDJ, Cleland LG, Cockshell MP, Mayrhofer Graham. Recruitment and proliferation of CD4+ T cells in synovium following adoptive transfer of adjuvant-induced arthritis. Int Immunol. 2006; 18(6):897-910. (Clone-specific: Flow cytometry, Immunofluorescence).
-
Starborg M, Gell K, Brundell E, Höög C. The murine Ki-67 cell proliferation antigen accumulates in the nucleolar and heterochromatic regions of interphase cells and at the periphery of the mitotic chromosomes in a process essential for cell cycle progression. J Cell Sci. 1996; 109(1):143-153. (Biology). View Reference
-
Valenti LM, Mathieu J, Chancerelle Y, et al. High levels of endogenous nitric oxide produced after burn injury in rats arrest activated T lymphocytes in the first G1 phase of the cell cycle and then induce their apoptosis. Exp Cell Res. 2005; 306(1):150-167. (Clone-specific: Flow cytometry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.