-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- Finland (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
BD IMag™ Anti-Human CD8 Magnetic Particles - DM
Clone SK1 (also known as Leu-2a; Leu2a) (RUO)
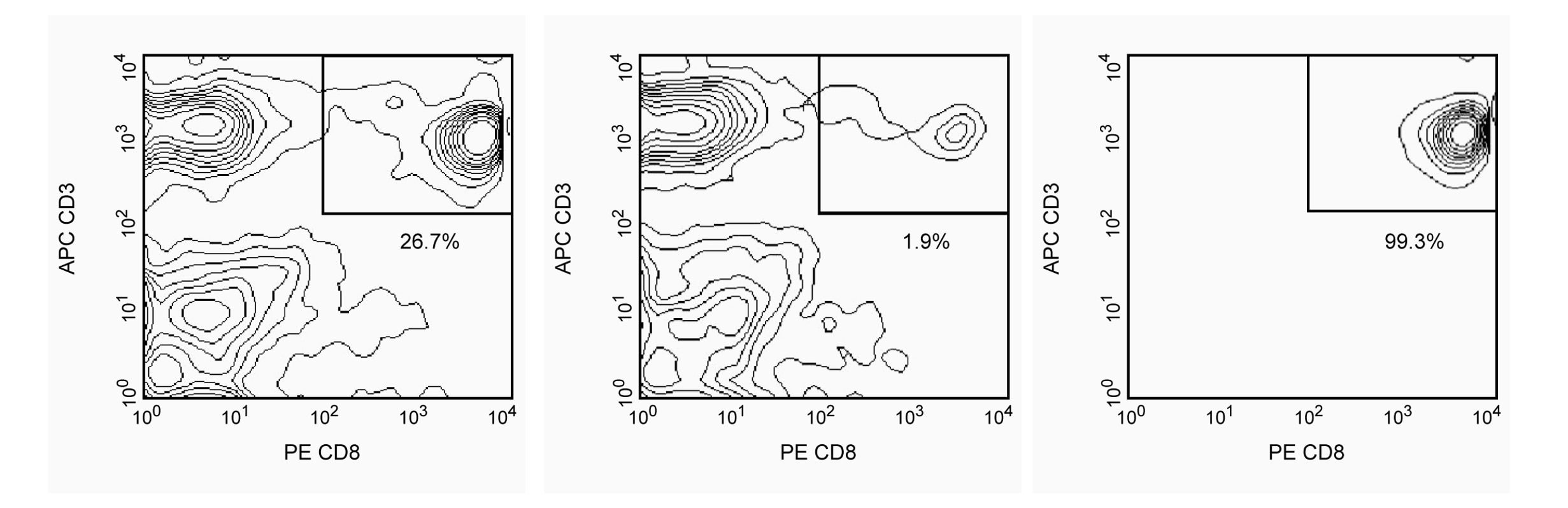
Positive selection of human CD8+ T lymphocytes from PBMC. Leukocytes were labeled with BD IMag™ Anti-Human CD8 Magnetic Particles - DM (Cat. No. 557766), separated using the BD IMag™ Cell Separation Magnet (Cat. No. 552311), and the negative (CD8-) and positive (CD8+) fractions were collected according to the accompanying protocol. Please refer to the Separation Flow Chart to identify the separated cell populations represented in this figure. For flow cytometric analysis, fresh PBMC (left panel), the negative fraction (middle panel) and the positive fraction (right panel) were stained with PE Mouse Anti-Human CD8 (Cat. No. 555367) and APC Mouse Anti-Human CD3 (Cat. No. 555335). The percent CD8+/CD3+ cells in each sample is given.


Positive selection of human CD8+ T lymphocytes from PBMC. Leukocytes were labeled with BD IMag™ Anti-Human CD8 Magnetic Particles - DM (Cat. No. 557766), separated using the BD IMag™ Cell Separation Magnet (Cat. No. 552311), and the negative (CD8-) and positive (CD8+) fractions were collected according to the accompanying protocol. Please refer to the Separation Flow Chart to identify the separated cell populations represented in this figure. For flow cytometric analysis, fresh PBMC (left panel), the negative fraction (middle panel) and the positive fraction (right panel) were stained with PE Mouse Anti-Human CD8 (Cat. No. 555367) and APC Mouse Anti-Human CD3 (Cat. No. 555335). The percent CD8+/CD3+ cells in each sample is given.

Positive selection of human CD8+ T lymphocytes from PBMC. Leukocytes were labeled with BD IMag™ Anti-Human CD8 Magnetic Particles - DM (Cat. No. 557766), separated using the BD IMag™ Cell Separation Magnet (Cat. No. 552311), and the negative (CD8-) and positive (CD8+) fractions were collected according to the accompanying protocol. Please refer to the Separation Flow Chart to identify the separated cell populations represented in this figure. For flow cytometric analysis, fresh PBMC (left panel), the negative fraction (middle panel) and the positive fraction (right panel) were stained with PE Mouse Anti-Human CD8 (Cat. No. 555367) and APC Mouse Anti-Human CD3 (Cat. No. 555335). The percent CD8+/CD3+ cells in each sample is given.



BD IMag™ Anti-Human CD8 Magnetic Particles - DM

BD IMag™ Anti-Human CD8 Magnetic Particles - DM

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
Peripheral Blood Mononuclear Cells (PBMC) are labeled with BD IMag™ Anti-Human CD8 Magnetic Particles - DM according to the Magnetic Labeling Protocol. This labeled cell suspension is then placed within the magnetic field of the BD IMag™ Cell Separation Magnet (Cat. No. 552311). Labeled cells migrate toward the magnet (positive fraction), leaving the unlabeled cells in suspension so they can be drawn off (negative fraction). The tube is then removed from the magnetic field for resuspension of the positive fraction. The separation is repeated twice to increase the purity of the positive fraction. The magnetic separation steps are diagrammed in the Separation Flow Chart. After the positive fraction is washed, the small size of the magnetic particles allows the positive fraction to be evaluated in downstream applications such as flow cytometry.
MAGNETIC LABELING PROTOCOL
1. Prepare PBMC from anti-coagulated human (or rhesus macaque) blood, preferably by density gradient centrifugation using Ficoll-Paque™.*
2. Dilute BD IMag™ Buffer (10X) (Cat. No. 552362) 1:10 with sterile distilled water or prepare 1X BD IMag™ buffer by supplementing Phosphate Buffered Saline with 0.5% BSA, 2 mM EDTA, and 0.09% sodium azide. Store at 4°C.
3. Count the cells, wash them with an excess volume of 1X BD IMag™ buffer, and carefully aspirate all the supernatant.
4. Vortex the BD IMag™ Anti-Human CD8 Magnetic Particles - DM thoroughly, and add 50 µl of particles for every 10^7 total cells.†
5. MIX THOROUGHLY. Incubate at room temperature for 30 minutes.‡
6. Bring the BD IMag™-particle labeling volume up to 1-8 x 10^7 cells/mL with 1X BD IMag™ buffer, and immediately place the tube on the Cell Separation Magnet. Incubate for 8-10 minutes.
7. With the tube on the Cell Separation Magnet, carefully aspirate off the supernatant. This supernatant contains the negative fraction.
8. Remove the tube from the Cell Separation Magnet, and add 1 ml of 1X BD IMag™ buffer to the same volume as in Step 6. Gently resuspend cells by pipetting up and down, and return the tube to the Cell Separation Magnet for another 2-4 minutes.
9. With the tube on the Cell Separation Magnet, carefully aspirate off the supernatant and discard.
10. Repeat Steps 8 and 9.
11. After the final wash step, resuspend the positive fraction in an appropriate buffer or media, and proceed with desired downstream application(s).
NOTES:
* Hints for successful cell preparation:
-Draw the blood into a tube containing EDTA.
-Remove the platelet rich plasma by centrifuging once at 220-240 × g.
-Wash 2-3 times in PBS after the density gradient separation.
-Remove clumps of cells and/or debris by passing the suspension through a 70-µm nylon cell strainer.
† The BD IMag™ particles may need to be titrated to optimize the separation of rhesus macaque leukocytes.
‡ Avoid nonspecific labeling by working quickly and adhering to the recommended incubation times.
Product Notices
- BD IMag™ particles are prepared from carboxy-functionalized magnetic particles which are manufactured by Skold Technology and are licensed under US patent number 7,169,618.
- Ficoll-Paque is a trademark of Amersham Biosciences Limited.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Data Sheets
Companion Products

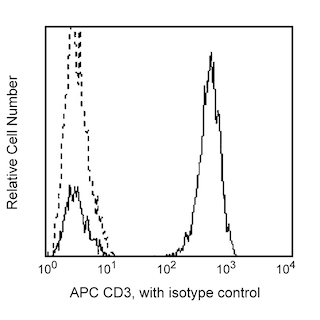

BD IMag™ anti-human CD8 Particles - DM are magnetic nanoparticles that have monoclonal antibody conjugated to their surfaces. These particles are optimized for the positive selection or depletion of CD8-bearing leukocytes using the BD IMag™ Cell Separation Magnet. CD8 is expressed on the peripheral MHC class I-restricted suppressor/cytotoxic T-lymphocyte subset, on a subset of NK cells, and on the majority of thymocytes. The SK1 mAb has been reported to cross-react with lymphocytes of chimpanzee and cynomolgus, pig-tailed, and rhesus macaque.
Development References (8)
-
Bahjat K. The Antibody Cross-Reactivity Resource. Available: www.keithbahjat.com/abcxr/ 2005.
-
Engleman EG, Benike CJ, Glickman E, Evans RL. Antibodies to membrane structures that distinguish suppressor/cytotoxic and helper T lymphocyte subpopulations block the mixed leukocyte reaction in man. J Exp Med. 1981; 154(1):193-198. (Biology). View Reference
-
Evans RL, Wall DW, Platsoucas CD, et al. Thymus-dependent membrane antigens in man: inhibition of cell-mediated lympholysis by monoclonal antibodies to TH2 antigen. Proc Natl Acad Sci U S A. 1981; 78(1):544-548. (Biology). View Reference
-
Kotzin BL, Benike CJ, Engleman EG. Induction of immunoglobulin-secreting cells in the allogeneic mixed leukocyte reaction: regulation by helper and suppressor lymphocyte subsets in man. J Immunol. 1981; 127(9):931-935. (Biology). View Reference
-
Lanier LL, Le AM, Phillips JH, Warner NL, Babcock GF. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983; 131(4):1789-1796. (Biology). View Reference
-
Ledbetter JA, Evans RL, Lipinski M, Cunningham-Rundles C, Good RA, Herzenberg LA. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981; 153(2):310-323. (Biology). View Reference
-
Ledbetter JA, Frankel AE, Herzenberg. Human Leu T-cell differentiation antigens: quantitative expression on normal lymphoid cells and cell lines. In: Hammerling G, Hammerling U, Kearney J, ed. Monoclonal Antibodies and T Cell Hybridomas: Perspectives and Technical News. New York: Elsevier/North Holland Biomedical Press; 1981:16-22.
-
Reichert T, DeBruyere M, Deneys V, et al. Lymphocyte subset reference ranges in adult Caucasians. Clin Immunol Immunopathol. 1991; 60(2):190-208. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.