Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


.png)

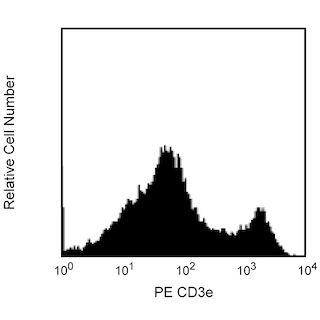
Differential expression of CD24 on thymocytes and peripheral T lymphocytes. C57BL/6 thymocytes were stained with FITC Rat anti-Mouse CD24 (Cat. No. 561777/553261, left panel). C57BL/6 splenocytes were simultaneously stained with FITC Rat anti-Mouse CD24 and PE Hamster anti-Mouse CD3e (Cat. No. 553063/553064, right panel). Flow cytometry was performed on a BD FACScan™ flow cytometry system.
.png)

BD Pharmingen™ FITC Rat Anti-Mouse CD24
.png)
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products
.png?imwidth=320)

The M1/69 monoclonal antibody specifically binds to CD24 (Heat-Stable Antigen, HSA or HsAg), a variably glycosylated, glycosyl-phosphatidylinositol-anchored membrane protein expressed on erythrocytes, granulocytes, monocytes, lymphocytes, and neurons. Hematopoietic stem cells of the embryonic yolk sac and fetal liver express CD24. Levels of expression of CD24 vary during differentiation of the T and B cell lineages. In the bone marrow, hematopoietic progenitors acquire CD24 expression upon commitment to the B-lymphocyte lineage. Immature B cells in the bone marrow express low CD24 levels whereas peripheral B lymphocytes express intermediate to high levels of CD24. The level of CD24 expression has been reported to rise upon activation of splenic B cells with LPS, but not with CD154 (CD40 Ligand). The majority of thymocytes express high levels of CD24, while most mature thymic and peripheral T lymphocytes do not express CD24. In contrast, TCR-bearing thymocytes which emigrate to the spleen are CD24+. Dendritic cells of the thymus, spleen, liver, and epidermal Langerhans cells have also been reported to express CD24. CD24 is not expressed by NK cells, as determined by staining with J11d mAb (Cat. No. 553146). CD24 is involved in the costimulation of CD4+ T cells by B cells, it is a "co-inducer" of in vitro thymocyte maturation, and it is a ligand of CD62P (P-selectin). While the monoclonal antibodies 30-F1, M1/69, and J11d all react with CD24, they show subtle differences in the level of staining of different lymphocyte populations. When possible, investigators should continue to use the same monoclonal anti-CD24 antibody as used in previous studies.
Development References (15)
-
Aigner S, Ruppert M, Hubbe M, et al. Heat stable antigen (mouse CD24) supports myeloid cell binding to endothelial and platelet P-selectin. Int Immunol. 1995; 7(10):1557-1565. (Biology). View Reference
-
Alterman LA, Crispe IN, Kinnon C. Characterization of the murine heat-stable antigen: an hematolymphoid differentiation antigen defined by the J11d, M1/69 and B2A2 antibodies. Eur J Immunol. 1990; 20(7):1597-1602. (Clone-specific). View Reference
-
Auerbach R, Huang H, Lu L. Hematopoietic stem cells in the mouse embryonic yolk sac. Stem Cells. 1996; 14(3):269-280. (Biology). View Reference
-
Calaora V, Chazal G, Nielsen PJ, Rougon G, Moreau H. mCD24 expression in the developing mouse brain and in zones of secondary neurogenesis in the adult. Neuroscience. 1996; 73(2):581-594. (Biology). View Reference
-
Cibotti R, Punt JA, Dash KS, Sharrow SO, Singer A. Surface molecules that drive T cell development in vitro in the absence of thymic epithelium and in the absence of lineage-specific signals. Immunity. 1997; 6(3):245-255. (Biology). View Reference
-
Crowley M, Inaba K, Witmer-Pack M, Steinman RM. The cell surface of mouse dendritic cells: FACS analyses of dendritic cells from different tissues including thymus. Cell Immunol. 1989; 118(1):108-125. (Clone-specific). View Reference
-
Hunte BE, Capone M, Zlotnik A, Rennick D, Moore TA. Acquisition of CD24 expression by Lin-CD43+B220(low)ckit(hi) cells coincides with commitment to the B cell lineage. Eur J Immunol. 1998; 28(11):3850-3856. (Biology). View Reference
-
Kelly KA, Pearse M, Lefrancois L, Scollay R. Emigration of selected subsets of gamma delta + T cells from the adult murine thymus. Int Immunol. 1993; 5(4):331-335. (Biology). View Reference
-
Kennedy MK, Mohler KM, Shanebeck KD, et al. Induction of B cell costimulatory function by recombinant murine CD40 ligand. Eur J Immunol. 1994; 24(1):116-123. (Biology). View Reference
-
Reichlin A, Iizuka K, Yokoyama WM. Isolation of murine natural killer cells. In: Coligan J, Kruisbeek AM, Margulies D, Shevach EM, Strober W, ed. Current Protocols in Immunology. New York: John Wiley and Sons; 1999:3.22.1-3.22.6.
-
Springer T, Galfre G, Secher DS, Milstein C. Monoclonal xenogeneic antibodies to murine cell surface antigens: identification of novel leukocyte differentiation antigens. Eur J Immunol. 1978; 8(8):539-551. (Immunogen). View Reference
-
Stall AM, Wells SM. FACS analysis of murine B-cell populations. In: Herzenberg LA, Weir DM, Blackwell C, ed. Weir's Handbook of Experimental Immunology. Blackwell Science Publishers; 1997:63.1-63.17.
-
Vremec D, Zorbas M, Scollay R, et al. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992; 176(1):47-58. (Biology). View Reference
-
Wenger RH, Rochelle JM, Seldin MF, Kohler G, Nielsen PJ. The heat stable antigen (mouse CD24) gene is differentially regulated but has a housekeeping promoter. J Biol Chem. 1993; 268(31):23345-23352. (Clone-specific). View Reference
-
Wilson A, Day LM, Scollay R, Shortman K. Subpopulations of mature murine thymocytes: properties of CD4-CD8+ and CD4+CD8- thymocytes lacking the heat-stable antigen. Cell Immunol. 1988; 117(2):312-326. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.