-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- BD OMICS-One™ WTA Next Assay
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?
BD Transduction Laboratories™ Purified Mouse Anti-DNA Ligase III
Clone 7/DNA Ligase III (RUO)
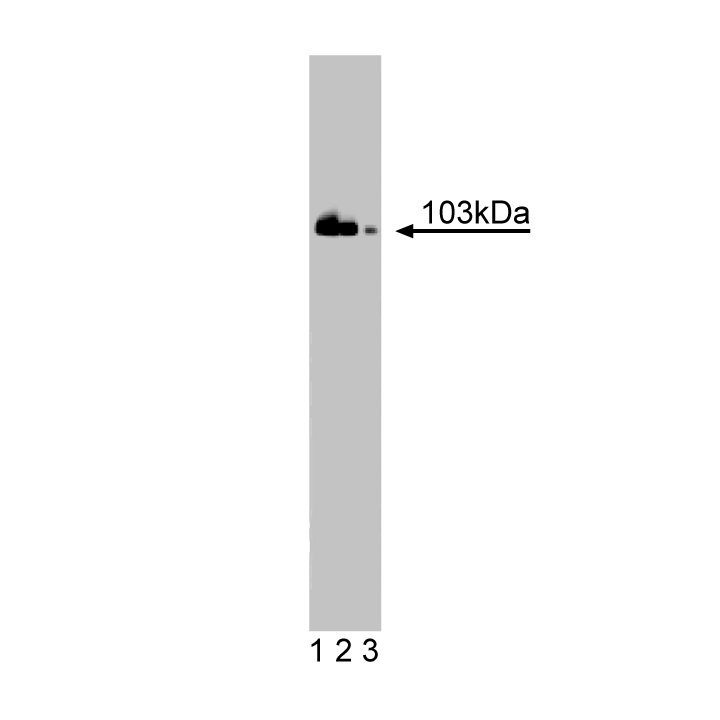



Western blot analysis of DNA Ligase III on Jurkat cell lysate. Lane 1: 1:10000, lane 2: 1:20000, lane 3: 1:40000 dilution of anti-DNA Ligase III.



Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
Cells have evolved DNA repair pathways that are dedicated to the maintenance of DNA integrity. In such pathways, damaged DNA is excised and the resulting gap is filled by DNA polymerase. Human DNA ligases, ligase I, III, and IV, utilize ATP as a co-factor in DNA joining reactions required for base excision and single strand break repair pathways. All DNA ligases contain an RFPR sequence and an active site motif (ASM) on each side of their catalytic domain. The RFPR is required for transfer of an AMP group from the enzyme to the 5'-phosphate terminus of a DNA nick. In addition, DNA ligase III has an N-terminal zinc finger domain (ZFD) that is homoloqous with the zinc fingers found in poly(ADP-ribose) polymerase (PARP). This domain is not required for DNA ligase activity, but enables DNA ligase III to interact with single strand gaps and single strand flaps. During base excision repair (BER), ATP-dependent ligation requires PARP, DNA polymerase β, and DNA ligase III interaction with XRCC1 within the BER complex. Thus, DNA ligase III may contain unique protein sequences that allow interaction and repair of specific types of DNA damage.
Development References (4)
-
Chen J, Tomkinson AE, Ramos W. Mammalian DNA ligase III: molecular cloning, chromosomal localization, and expression in spermatocytes undergoing meiotic recombination. Mol Cell Biol. 1995; 15(10):5412-5422. (Biology). View Reference
-
Mackey ZB, Niedergang C, Murcia JM. DNA ligase III is recruited to DNA strand breaks by a zinc finger motif homologous to that of poly(ADP-ribose) polymerase. Identification of two functionally distinct DNA binding regions within DNA ligase III. J Biol Chem. 1999; 274(31):21679-21687. (Biology). View Reference
-
Oei SL, Ziegler M. ATP for the DNA ligation step in base excision repair is generated from poly(ADP-ribose). J Biol Chem. 2000; 275(30):23234-23239. (Biology). View Reference
-
Taylor RM, Whitehouse CJ, Caldecott KW. The DNA ligase III zinc finger stimulates binding to DNA secondary structure and promotes end joining. Nucleic Acids Res. 2000; 28(18):3558-3563. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.