Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
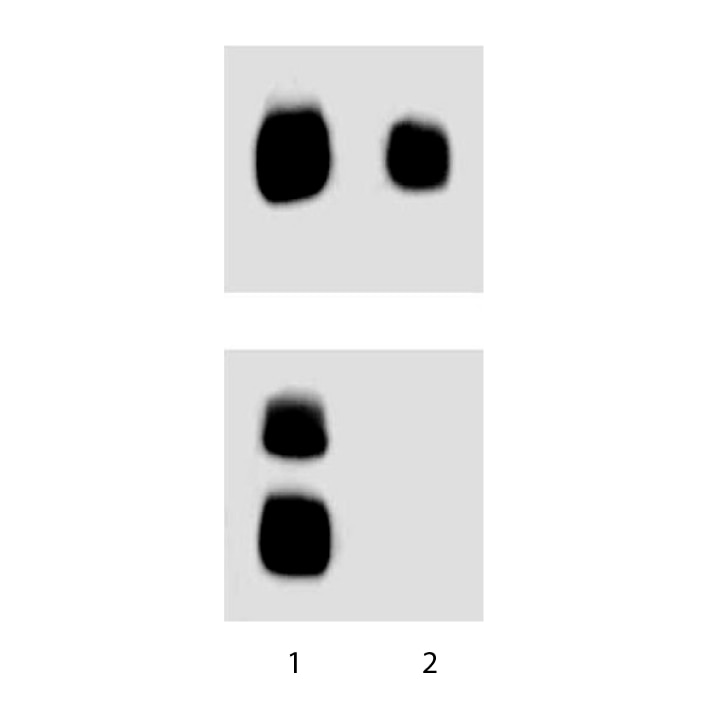




RSV-3T3 lysate was either left untreated (lane 1) or treated (lane 2) with 50 µg/ml alkaline phosphatase for 30 minutes at 37°C. The top panel was probed with an anti-GSK-3β (Cat. No. 610201) antibody and the bottom was probed with the anti-GSK-3β (pY216) antibody (Cat. No. 612312) at a 1:1000 dilution.

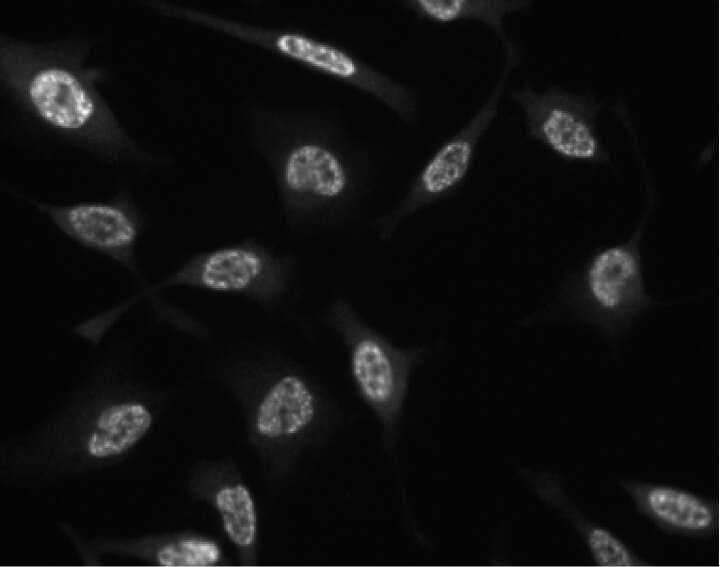
Immunofluorescent staining of HeLa (ATCC CCL-2) cells. Cells were seeded in a 96 well imaging plate (Cat. No. 353219) at ~10 000 cells per well. After overnight incubation, cells were stained using the alcohol perm protocol and the anti-GSK-3β (pY216) antibody. The second step reagent was Alexa Fluor® 555 anti-mouse IgG (Invitrogen). The image was taken on a BD Pathway™ 855 Bioimager using a 20x objective. This antibody also stained A549 (ATCC CCL-185) and U-2 OS (ATCC HTB-96) cells and worked with both the Triton™ X-100 and allcohol perm protocols (see Recommended Assay Procedure).


BD Transduction Laboratories™ Purified Mouse Anti-GSK-3β (pY216)

BD Transduction Laboratories™ Purified Mouse Anti-GSK-3β (pY216)

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
Bioimaging
1. Seed the cells in appropriate culture medium at ~10,000 cells per well in a BD Falcon™ 96-well Imaging Plate (Cat. No. 353219) and culture overnight.
2. Remove the culture medium from the wells, and fix the cells by adding 100 μl of BD Cytofix™ Fixation Buffer (Cat. No. 554655) to each well. Incubate for 10 minutes at room temperature (RT).
3. Remove the fixative from the wells, and permeabilize the cells using either BD Perm Buffer III, 90% methanol, or Triton™ X-100:
a. Add 100 μl of -20°C 90% methanol or Perm Buffer III (Cat. No. 558050) to each well and incubate for 5 minutes at RT.
OR
b. Add 100 μl of 0.1% Triton™ X-100 to each well and incubate for 5 minutes at RT.
4. Remove the permeabilization buffer, and wash the wells twice with 100 μl of 1× PBS.
5. Remove the PBS, and block the cells by adding 100 μl of BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656) to each well. Incubate for 30 minutes at RT.
6. Remove the blocking buffer and add 50 μl of the optimally titrated primary antibody (diluted in Stain Buffer) to each well, and incubate for 1 hour at RT.
7. Remove the primary antibody, and wash the wells three times with 100 μl of 1× PBS.
8. Remove the PBS, and add the second step reagent at its optimally titrated concentration in 50 μl to each well, and incubate in the dark for 1 hour at RT.
9. Remove the second step reagent, and wash the wells three times with 100 μl of 1× PBS.
10. Remove the PBS, and counter-stain the nuclei by adding 200 μl per well of 2 μg/ml Hoechst 33342 (e.g., Sigma-Aldrich Cat. No. B2261) in 1× PBS to each well at least 15 minutes before imaging.
11. View and analyze the cells on an appropriate imaging instrument.
Bioimaging: For more detailed information please refer to http://www.bdbiosciences.com/support/resources/protocols/ceritifed_reagents.jsp
Western blot: For more detailed information please refer to http://www.bdbiosciences.com/pharmingen/protocols/Western_Blotting.shtml
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- This antibody has been developed and certified for the bioimaging application. However, a routine bioimaging test is not performed on every lot. Researchers are encouraged to titrate the reagent for optimal performance.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Triton is a trademark of the Dow Chemical Company.
Companion Products



Glycogen Synthase Kinase-3β (GSK-3β) is a serine/threonine kinase that affects glycogen metabolism by phosphorylating and down-regulating the activity of muscle glycogen synthase. GSK-3β is identical to the Tau Protein Kinase I (TPK I) that plays a role in the formation of the histopathological brain lesions of Alzheimer's disease (AD). Phosphorylation of the cytoskeletal protein, tau, by GSK-3β converts these proteins into paired helical filaments (PHF) which are found in the neurofibrillary tangles and degenerative neurites of AD patients. Regulation of GSK-3β activity through both serine and tyrosine phosphoylation is a critical determinant of cell death or survival. Factors that promote cell survival, such as growth factors, activate Akt which, in turn phosphorylates GSK-3β at Ser-9, leading to inactivation of its kinase activity. On the contrary, events that promote cell death, such as growth factor removal, cause increases in phosphorylation within the catalytic domain at Tyr-216 and stimulate kinase activity. Thus GSK-3β is a tightly regulated death promoting kinase that regulates the activity of various proteins, including cytokeletal and enzymatic proteins.
Development References (2)
-
Bhat RV, Shanley J, Correll MP, et al. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc Natl Acad Sci U S A. 2000; 97(20):11074-11079. (Biology). View Reference
-
Hartigan JA, Johnson GV. Transient increases in intracellular calcium result in prolonged site-selective increases in Tau phosphorylation through a glycogen synthase kinase 3beta-dependent pathway. J Biol Chem. 1999; 274(30):21395-21401. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.