Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
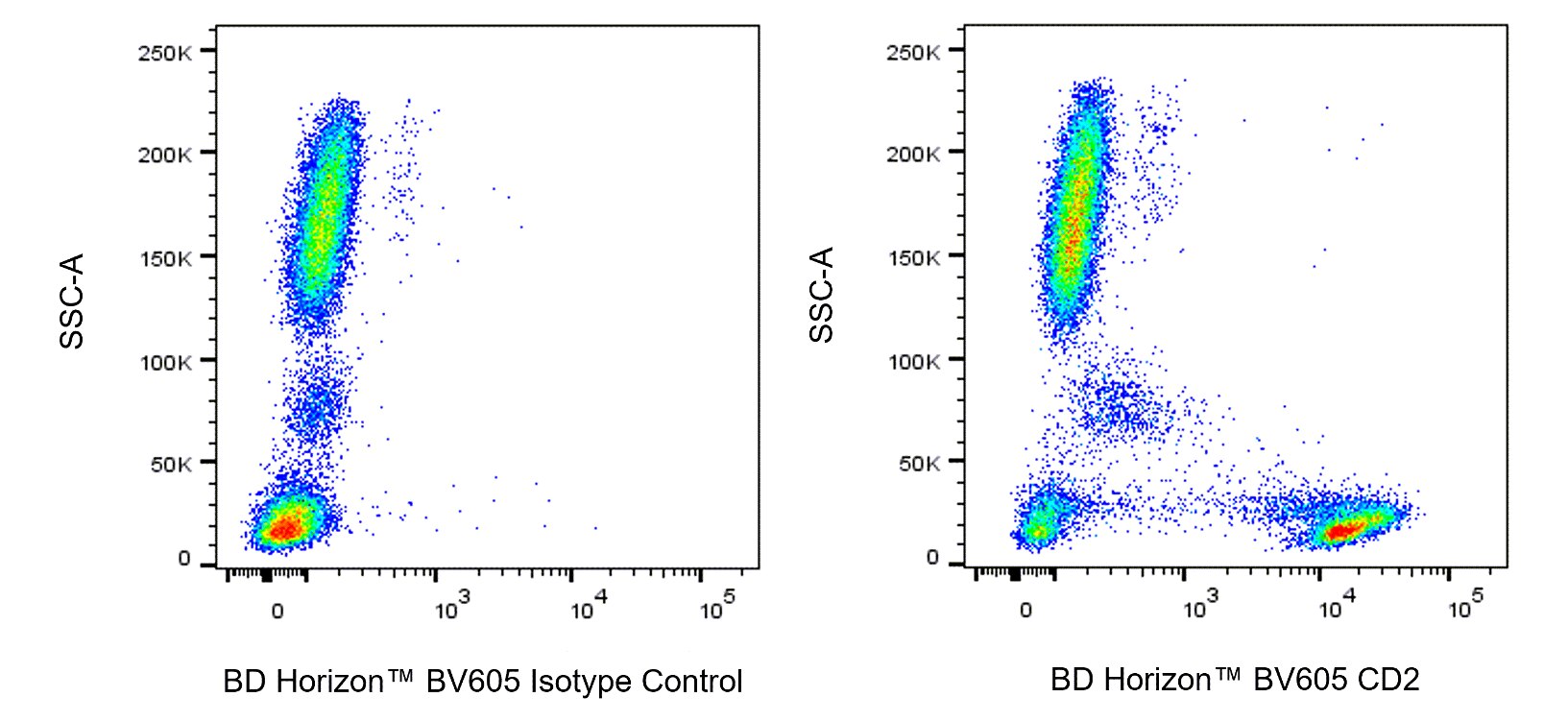



Multiparameter flow cytometric analysis of CD2 expression on Human peripheral blood leucocyte populations. Human whole blood was stained with either BD Horizon™ BV605 Mouse IgG1, κ Isotype Control (Cat. No. 562652; Left Plot) or BD Horizon™ BV605 Mouse Anti-Human CD2 antibody (Cat. No. 569168; Right Plot) at 0.125 µg/test. Erythrocytes were lysed with BD FACS™ Lysing Solution (Cat. No. 349202). The bivariate pseudocolor density plot showing the correlated expression of CD2 (or Ig Isotype control staining) versus side-light scatter (SSC-A) signals was derived from gated events with the forward and side light-scatter characteristics of intact leucocytes. Flow cytometry and data analysis were performed using a BD LSRFortessa™ X-20 Cell Analyzer System and FlowJo™ software. Data shown on this Technical Data Sheet are not lot specific.


BD Horizon™ BV605 Mouse Anti-Human CD2

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
BD® CompBeads can be used as surrogates to assess fluorescence spillover (compensation). When fluorochrome conjugated antibodies are bound to BD® CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cells and BD® CompBeads to ensure that BD® CompBeads are appropriate for your specific cellular application.
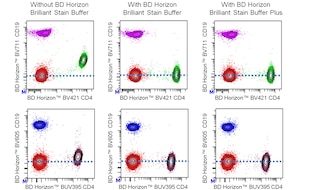
For optimal and reproducible results, BD Horizon Brilliant™ Stain Buffer should be used anytime BD Horizon Brilliant dyes are used in a multicolor flow cytometry panel. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. The BD Horizon Brilliant Stain Buffer was designed to minimize these interactions. When BD Horizon Brilliant Stain Buffer is used in in the multicolor panel, it should also be used in the corresponding compensation controls for all dyes to achieve the most accurate compensation. For the most accurate compensation, compensation controls created with either cells or beads should be exposed to BD Horizon Brilliant Stain Buffer for the same length of time as the corresponding multicolor panel. More information can be found in the Technical Data Sheet of the BD Horizon Brilliant Stain Buffer (Cat. No. 563794/566349) or the BD Horizon Brilliant Stain Buffer Plus (Cat. No. 566385).
Product Notices
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- An isotype control should be used at the same concentration as the antibody of interest.
- Please observe the following precautions: Absorption of visible light can significantly alter the energy transfer occurring in any tandem fluorochrome conjugate; therefore, we recommend that special precautions be taken (such as wrapping vials, tubes, or racks in aluminum foil) to prevent exposure of conjugated reagents, including cells stained with those reagents, to room illumination.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Although every effort is made to minimize the lot-to-lot variation in the efficiency of the fluorochrome energy transfer, differences in the residual emission from BD Horizon™ BV421 may be observed. Therefore, we recommend that individual compensation controls be performed for every BD Horizon™ BV605 conjugate.
- BD Horizon Brilliant Violet 605 is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,227,187; 8,455,613; 8,575,303; 8,354,239.
- BD Horizon Brilliant Stain Buffer is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,575,303; 8,354,239.
- Human donor specific background has been observed in relation to the presence of anti-polyethylene glycol (PEG) antibodies, developed as a result of certain vaccines containing PEG, including some COVID-19 vaccines. We recommend use of BD Horizon Brilliant™ Stain Buffer in your experiments to help mitigate potential background. For more information visit https://www.bdbiosciences.com/en-us/support/product-notices.
- Species cross-reactivity detected in product development may not have been confirmed on every format and/or application.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- CF™ is a trademark of Biotium, Inc.
- For U.S. patents that may apply, see bd.com/patents.
Companion Products


The RPA-2.10 monoclonal antibody specifically binds to CD2 which is also known as Lymphocyte-function antigen-2 (LFA-2), LFA-3 receptor, Erythrocyte receptor, Sheep red blood cell (SRBC) receptor, or T-cell surface antigen T11/Leu-5. CD2 is a 50 kDa type I transmembrane glycoprotein. CD2 belongs to the immunoglobulin superfamily of proteins along with its primary ligand, LFA-3 (CD58). It is expressed on the surface of ~80-90% of human peripheral blood lymphocytes, greater than 95% of thymocytes, all T lymphocytes that form E-rosettes, and a subset of NK cells. CD2 functions as an adhesion receptor that binds to CD58 resulting in the activation of CD2-positive T cells and NK cells and in the regulation of their cytolytic activities.
Development References (8)
-
Aversa GG, Bishop GA, Suranyi MG, Hall BM. RPA-2.10: an anti-CD2 monoclonal antibody that inhibits alloimmune responses and monitors T cell activation. Transplant Proc. 1987; 19(1):277-278. (Immunogen: Flow cytometry, Immunoprecipitation, Inhibition). View Reference
-
Bjornson-Hooper ZB, Fragiadakis GK, Spitzer MH, et al. A Comprehensive Atlas of Immunological Differences Between Humans, Mice, and Non-Human Primates.. Front Immunol. 2022; 13:867015. (Clone-specific: Flow cytometry). View Reference
-
Hahn WC, Burakoff SJ, Bierer BE. Signal transduction pathways involved in T cell receptor-induced regulation of CD2 avidity for CD58. J Immunol. 1993; 150(7):2607-2619. (Biology). View Reference
-
Jonker M, Slingerland W. Reactivity of mAb specific for human CD markers with Rhesus monkey leucocytes. In: Knapp W. W. Knapp .. et al., ed. Leucocyte typing IV : white cell differentiation antigens. Oxford New York: Oxford University Press; 1989:1058-1063.
-
Kato K. CD2 Workshop Panel report. In: Kishimoto T. Tadamitsu Kishimoto .. et al., ed. Leucocyte typing VI : white cell differentiation antigens : proceedings of the sixth international workshop and conference held in Kobe, Japan, 10-14 November 1996. New York: Garland Pub.; 1997:39-43.
-
Knapp W. W. Knapp .. et al., ed. Leucocyte typing IV : white cell differentiation antigens. Oxford New York: Oxford University Press; 1989:1-1182.
-
Sopper S, Stahl-Hennig C, Demuth M, Johnston IC, Dorries R, ter Meulen V. Lymphocyte subsets and expression of differentiation markers in blood and lymphoid organs of rhesus monkeys. Cytometry. 1997; 29(4):351-362. (Clone-specific: Flow cytometry). View Reference
-
Suranyi MG, Bishop GA, Clayberger C, et al. Lymphocyte adhesion molecules in T cell-mediated lysis of human kidney cells. Kidney Int. 1991; 39(2):312-319. (Clone-specific: Flow cytometry, Inhibition). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.