Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


CD61 FITC
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
The antibody reagent is stable until the expiration date shown on the label when stored at 2° to 8°C. Do not use after the expiration date. Do not freeze the reagent or expose it to direct light during storage or incubation with cells. Keep the outside of the reagent vial dry.
Do not use the reagent if you observe any change in appearance. Precipitation or discoloration indicates instability or deterioration.
CD61 is intended for in vitro diagnostic use in the identification of cells expressing CD61 antigen, using a BD FACS™ brand flow cytometer. The flow cytometer must be equipped to detect light scatter and the appropriate fluorescence, and be equipped with appropriate analysis software (such as BD CellQuest™ or BD LYSYS™ II software) for data acquisition and analysis. Refer to your instrument user’s guide for instructions.
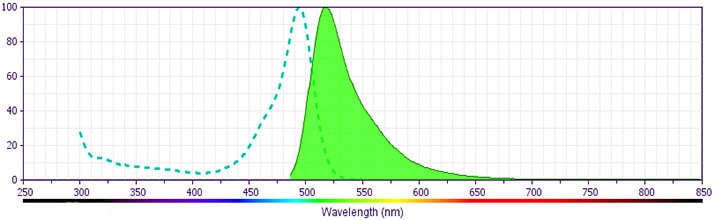
Development References (21)
-
Bene MC, Castoldi G, Knapp W, et al. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia. 1995; 9(10):1783-1786. (Biology).
-
Centers for Disease Control. Update: universal precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other bloodborne pathogens in healthcare settings. MMWR. 1988; 37:377-388. (Biology).
-
Clinical Applications of Flow Cytometry: Quality Assurance and Immunophenotyping of Lymphocytes: Approved Guideline. H42-A2. 2007. (Biology).
-
Consensus protocol for the flow cytometric immunophenotyping of hematopoietic malignancies. Rothe G, Schmitz G. Leukemia. 1996; 10:877-895. (Biology).
-
Erber WN, Breton-Gorius J, Villeval JL, Oscier DG, Bai Y, Mason DY. Detection of cells of magakaryocyte lineage in haematological malignancies by immuno-alkaline phosphatase labelling cell smears with a panel of monoclonal antibodies. Br J Haematol. 1987; 65:87-94. (Biology).
-
Fijnheer R, Modderman PW, Veldman H, et al. Detection of platelet activation with monoclonal antibodies and flow cytometry. Changes during platelet storage.. Transfusion. 1990; 30(1):20-5. (Biology). View Reference
-
Fitzgerald LA, Steiner B, Rall SC, Lo SS, Phillips DR. Protein sequence of endothelial glycoprotein IIIa derived from a cDNA clone. Identity with platelet glycoprotein IIIa and similarity to "integrin".. J Biol Chem. 1987; 262(9):3936-9. (Biology). View Reference
-
Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992; 69(1):11-25. (Biology). View Reference
-
Jackson AL, Warner NL. Rose NR, Friedman H, Fahey JL, ed. Manual of Clincial Laboratory Immunology, Third Edition. Washington DC: American Society for Microbiology; 1986:226-235.
-
Jennings LK, Ashmun RA, Wang WC, Dockter ME. Analysis of human platelet glycoproteins IIb-IIIa and Glanzmann's thrombasthenia in whole blood by flow cytometry.. Blood. 1986; 68(1):173-9. (Biology). View Reference
-
Modderman PW. Knapp W, Dörken B, Gilks WR, et al, ed. Leucocyte Typing IV: White Cell Differentiation Antigens. 1989:1017-1019.
-
Modderman PW. Knapp W, Dörken B, Gilks WR, et al, ed. Leucocyte Typing IV: White Cell Differentiation Antigens. New York, NY: Oxford University Press; 1989:1025.
-
NCCLS document. 2001. (Biology).
-
Parmentier S, Kaplan C, Catimel B, McGregor JL. New families of adhesion molecules play a vital role in platelet functions.. Immunol Today. 1990; 11(7):225-7. (Biology). View Reference
-
Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation.. J Biol Chem. 1985; 260(20):11107-14. (Biology). View Reference
-
Springer TA. Adhesion receptors of the immune system. Nature. 1990; 346(6283):425-434. (Biology). View Reference
-
Stelzer GT, Marti G, Hurley A, McCoy PJ, Lovett EJ, Schwartz A. US-Canadian consensus recommendations on the immunophenotypic analysis of hematologic neoplasia by flow cytometry: standardization and validation of laboratory procedures. Cytometry. 1997; 30:214-230. (Biology).
-
Wong DA, Springer TA. Schlossman SF, Boumsell L, Gilks W, et al, ed. Leucocyte Typing V: White Cell Differentiation Antigens. New York, NY: Oxford University Press; 1995:1664-1665.
-
von dem Borne AEGKr, Admiraal LG, Daams M, Hogervorst F, Modderman PW. Knapp W, Dörken B, Gilks WR, et al, ed. Leucocyte Typing IV: White Cell Differentiation Antigens. New York, NY: Oxford University Press; 1989:967-969.
-
von dem Borne AEGKr, Modderman PW, Admiraal LG, Nieuwenhuis, HK. Platelet antibodies, the overall results. In: Knapp W. W. Knapp .. et al., ed. Leucocyte typing IV : white cell differentiation antigens. Oxford New York: Oxford University Press; 1989:951-966.
-
von dem Borne AEGKr, Modderman PW. Knapp W, Dörken B, Gilks WR, et al, ed. Leucocyte Typing IV: White Cell Differentiation Antigens. New York, NY: Oxford University Press; 1989:997-999.
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For In Vitro Diagnostic Use.
23-22942-00
![]()
Documents are subject to revision without notice. Please verify you have the correct revision of the document, and always refer back to BD's eIFU website for the latest and most up to date information.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.