Standardised Flow Cytometry, a Key Analytical Platform in Cell Therapy Manufacturing
Webinar by Gert Boschman, European Scientific Affairs Director, BD Biosciences
January 03, 2022
The past two years have seen the “cell therapy revolution” begin. It now sees the patient’s cells being modified to fight a disease. This revolution brings along unique challenges from discovery through clinical trials to the clinic. An evolving regulatory landscape, rapid advances in manufacturing technologies, products with complex mechanism of action and inherent variability in starting material are just a few examples of these challenges. Another important challenge to commercialising cell-based therapies is developing scalable and transferable manufacturing process while maintaining the product attributes.
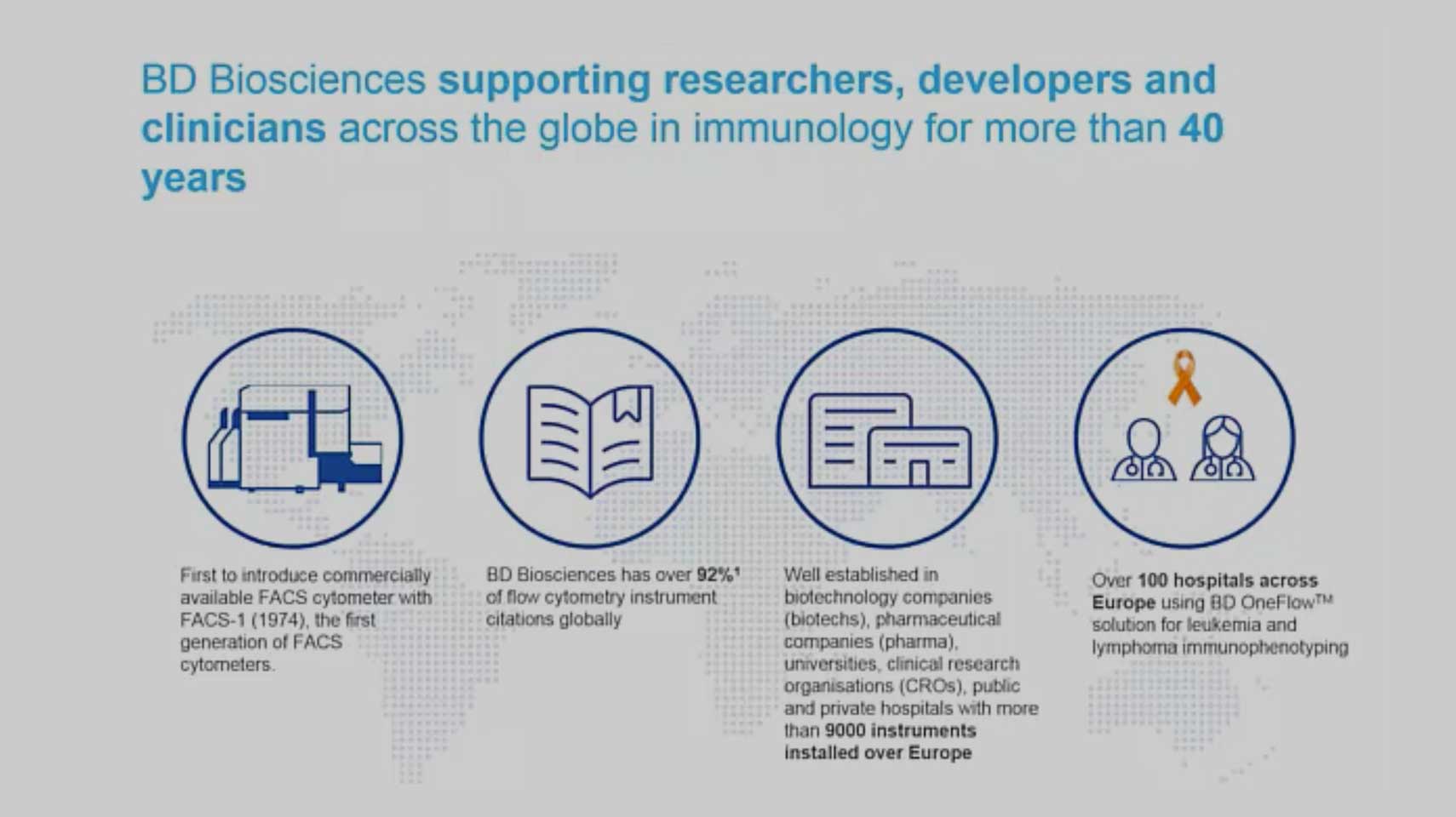
In this webinar, Gert Boschman, European Scientific Affairs Director, BD Biosciences talks about how BD is enabling clinical researchers to overcome the challenges posed by cell therapy. The BD cell characterisation solutions span the entire product development continuum. They include ways to standardise instrument setup, automate sample preparation and deepen the biological understanding of your product.