Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?




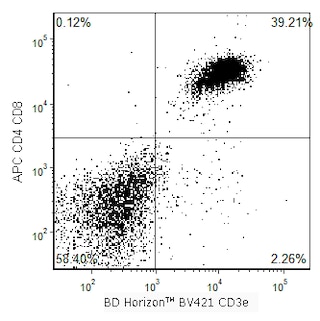
Two-color flow cytometric analysis of CD8 expression on mouse splenocytes. Mouse splenic leucocytes were preincubated with Purified Rat Anti-Mouse CD16/32 antibody (Mouse Fc Block™) Cat. No. 553141/553142). The cells were then stained with BD Horizon™ BV421 Hamster Anti-Mouse CD3e antibody (Cat. No. 562600) and with either BD Horizon™ RY586 Rat IgG2a, κ Isotype Control (Cat. No. 568130; Left Plot) or BD Horizon™ RY586 Rat Anti-Mouse CD8a antibody (Cat. No. 568163/568164; Right Plot) at 0.5 µg/test. The two-color fluorescence contour plot showing the correlated expression of CD4 (or Ig Isotype control staining) versus CD3e was derived from gated events with the forward and side light-scatter characteristic of viable splenic leucocytes. Flow cytometry and data analysis were performed using a BD FACSymphony™ A5 SE Flow Cytometer and FlowJo™ software. Data shown on this Technical Data Sheet are not lot specific.


BD Horizon™ RY586 Rat Anti-Mouse CD8a

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
BD® CompBeads can be used as surrogates to assess fluorescence spillover (compensation). When fluorochrome conjugated antibodies are bound to BD® CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cells and BD® CompBeads to ensure that BD® CompBeads are appropriate for your specific cellular application.
Product Notices
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- An isotype control should be used at the same concentration as the antibody of interest.
- CF™ is a trademark of Biotium, Inc.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
Companion Products



The 53-6.7 monoclonal antibody specifically binds to the 38 kDa α and 34 kDa α' chains of the CD8 differentiation antigen (Ly-2 or Lyt-2) of all mouse strains tested. The CD8 α and α' chains (CD8a) form heterodimers with the CD8 β chain (CD8b, Ly-3, or Lyt-3) on the surface of most thymocytes. A subpopulation of mature T lymphocytes (i.e., MHC class I-restricted T cells, including most T suppressor/cytotoxic cells) expresses almost exclusively the CD8 αβ heterodimer. Subsets of γδ TCR-bearing T cells, intestinal intrapithelial lymphocytes, and dendritic cells express CD8a without CD8b. It has been suggested that the expression of the CD8a/CD8b heterodimer is restricted to T lymphocytes which matured in the thymus or in an extrathymic environment that had been influenced by thymus-initiated neuroendocrine signals. CD8 is an antigen coreceptor on the T-cell surface which interacts with MHC class I molecules on antigen-presenting cells or epithelial cells. It participates in T-cell activation through its association with the T-cell receptor complex and protein tyrosine kinase lck (p56 [lck]). The CD8 α and α' chains arise from alternatively spliced messengers of a single CD8a gene. The longer α form associates with p56 [lck] via a CXCP motif in its cytoplasmic domain, which it shares with CD4, but not with CD8b. The truncated α' chain is unable to associate with p56 [lck], and it may function to attenuate the CD8-mediated costimulatory signal during intrathymic T-cell maturation. In vivo and in vitro treatment with 53-6.7 mAb has reportedly been effective at depleting CD8+ peripheral T lymphocytes. The 53-6.7 antibody has also been reported to cross-react with CD8 α- and α'-like polypeptides on subsets of thymic and peripheral lymphocytes in the Egyptian toad, Bufo regularis.
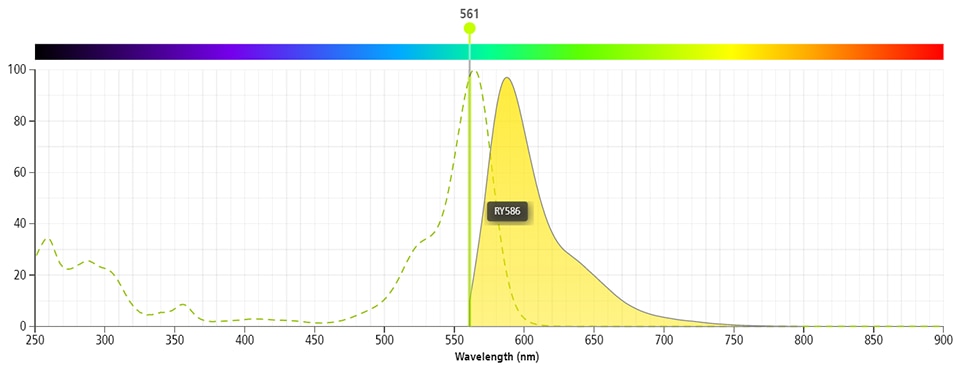
The BD Horizon RealYellow™ 586 (RY586) Dye is part of the BD family of yellow-green dyes. It is a small organic fluorochrome with an excitation maximum (Ex Max) at 565-nm and an emission maximum (Em Max) at 586-nm. Driven by BD innovation, RY586 can be used on both spectral and conventional cytometers and is designed to be excited by the Yellow-Green laser (561-nm) with minimal excitation by the 488-nm Blue laser. For conventional instruments equipped with a Yellow-Green laser (561-nm), RY586 can be used as an alternative to PE and we recommend using an optical filter centered near 586-nm (eg, a 586/15-nm bandpass filter). For spectral instruments equipped with a Yellow-Green laser (561-nm), it can be used in conjunction with PE. Compared to PE, RY586 is similar in brightness, minimal spillover into Blue detectors, and increased spillover into the 610/20-nm (PE-CF594) detector. Please ensure that your instrument configuration (lasers and optical filters) is appropriate for this dye.
Development References (14)
-
Fujiura Y, Kawaguchi M, Kondo Y, et al. Development of CD8 alpha alpha+ intestinal intraepithelial T cells in beta 2-microglobulin- and/or TAP1-deficient mice. J Immunol. 1996; 156(8):2710-2715. (Clone-specific: Flow cytometry). View Reference
-
Hathcock KS. T cell depletion by cytotoxic elimination. Curr Protoc Immunol. 1991; 1:3.4.1-3.4.3. (Clone-specific: Cell separation, Depletion, Flow cytometry). View Reference
-
Kruisbeek AM, Shevach EM. Proliferative assays for T cell function. Curr Protoc Immunol. 2004; 3:3.12.1-3.12.14. (Clone-specific: Depletion, In vivo exacerbation). View Reference
-
LeFrancois L. Extrathymic differentiation of intraepithelial lymphocytes: generation of a separate and unequal T-cell repertoire. Immunol Today. 1991; 12(12):436-438. (Biology). View Reference
-
Ledbetter JA, Herzenberg LA. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979; 47:63-90. (Immunogen: Flow cytometry, Immunofluorescence, Immunoprecipitation). View Reference
-
Ledbetter JA, Rouse RV, Micklem HS, Herzenberg LA. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980; 152(2):280-295. (Immunogen: Flow cytometry). View Reference
-
Ledbetter JA, Seaman WE, Tsu TT, Herzenberg LA. Lyt-2 and lyt-3 antigens are on two different polypeptide subunits linked by disulfide bonds. Relationship of subunits to T cell cytolytic activity. J Exp Med. 1981; 153(6):1503-1516. (Clone-specific: Blocking, Flow cytometry, Immunoprecipitation, Inhibition). View Reference
-
Leishman AJ, Naidenko OV, Attinger A, et al. T cell responses modulated through interaction between CD8alphaalpha and the nonclassical MHC class I molecule, TL. Science. 2001; 294(5548):1848-1849. (Clone-specific: Blocking, Flow cytometry). View Reference
-
MacDonald HR, Schreyer M, Howe RC, Bron C. Selective expression of CD8 alpha (Ly-2) subunit on activated thymic gamma/delta cells. Eur J Immunol. 1990; 20(4):927-930. (Biology). View Reference
-
Nakayama K, Nakayama K, Negishi I, et al. Requirement for CD8 beta chain in positive selection of CD8-lineage T cells. Science. 1994; 263(5150):1131-1133. (Biology). View Reference
-
Sydora BC, Brossay L, Hagenbaugh A, Kronenberg M, Cheroutre H. TAP-independent selection of CD8+ intestinal intraepithelial lymphocytes. J Immunol. 1996; 156(11):4209-4216. (Clone-specific: Flow cytometry). View Reference
-
Takahashi K, Nakata M, Tanaka T, et al. CD4 and CD8 regulate interleukin 2 responses of T cells. Proc Natl Acad Sci U S A. 1992; 89(12):5557-5561. (Clone-specific: Immunoprecipitation, Inhibition). View Reference
-
Vremec D, Zorbas M, Scollay R, et al. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992; 176(1):47-58. (Clone-specific: Flow cytometry). View Reference
-
van Ewijk W, van Soest PL, van den Engh GJ. Fluorescence analysis and anatomic distribution of mouse T lymphocyte subsets defined by monoclonal antibodies to the antigens Thy-1, Lyt-1, Lyt-2, and T-200. J Immunol. 1981; 127(6):2594-2604. (Clone-specific: Flow cytometry, Immunofluorescence, Immunohistochemistry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.