Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
BD® CompBeads can be used as surrogates to assess fluorescence spillover (compensation). When fluorochrome conjugated antibodies are bound to BD® CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cells and BD® CompBeads to ensure that BD® CompBeads are appropriate for your specific cellular application.
Product Notices
- Researchers should determine the optimal concentration of this reagent for their individual applications.
- The production process underwent stringent testing and validation to assure that it generates a high-quality conjugate with consistent performance and specific binding activity. However, verification testing has not been performed on all conjugate lots.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- CF™ is a trademark of Biotium, Inc.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Human donor specific background has been observed in relation to the presence of anti-polyethylene glycol (PEG) antibodies, developed as a result of certain vaccines containing PEG, including some COVID-19 vaccines. We recommend use of BD Horizon Brilliant™ Stain Buffer in your experiments to help mitigate potential background. For more information visit https://www.bdbiosciences.com/en-us/support/product-notices.
Companion Products


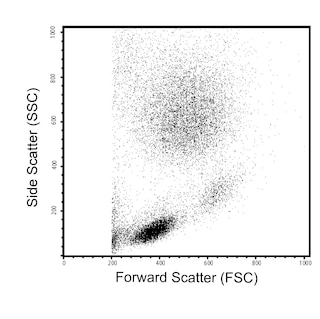

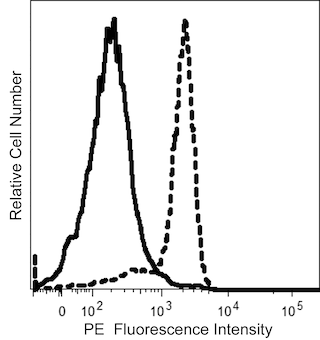

The 6H6 monoclonal antibody specifically recognizes the Interleukin-3 receptor alpha chain (IL-3Ra) which is also known as CD123. IL-3Ra (CD123) is a ~70 kDa type I transmembrane glycoprotein that is encoded by IL3RA (interleukin 3 receptor subunit alpha) which belongs to the type I cytokine receptor family within the immunoglobulin gene superfamily. This receptor chain consists of an extracellular region that contains an immunoglobulin-like N-terminal domain (NTD) with a fibronectin type III (FnIII) fold followed by two more FnIII domains that form the cytokine receptor module (CRM), a transmembrane region, and an intracellular tail. IL-3Ra (CD123) binds IL-3 specifically and with low affinity. IL-3Ra (CD123) forms a high-affinity signaling receptor for IL-3 (IL-3R) with the ß common chain (ßc; also known as, CD131) that is shared with the heterodimeric IL-5 and granulocyte-macrophage colony-stimulating factor (GM-CSF) receptors. IL-3Ra (CD123) is variably expressed on certain hematopoietic progenitor cells, basophils, eosinophils, mast cells, monocytes, macrophages, dendritic cells, megakaryocytes, and on some B cells, endothelial cells, and leukemia cells. Bound IL-3 can signal through IL-3R to promote the activation, proliferation, differentiation, and viability of these cells. Amongst monoclonal antibodies specific for human IL-3Ra (CD123), the 6H6 and 9F5 antibodies do not block IL-3 binding to the IL-3R whereas the 7G3 antibody does block IL-3 binding to its receptor in a dose-dependent manner.
Development References (7)
-
Broughton SE, Hercus TR, Hardy MP, et al. Dual mechanism of interleukin-3 receptor blockade by an anti-cancer antibody.. Cell Rep. 2014; 8(2):410-9. (Biology). View Reference
-
Kohrgruber N, Halanek N, Gröger M, et al. Survival, maturation, and function of CD11c- and CD11c+ peripheral blood dendritic cells are differentially regulated by cytokines.. J Immunol. 1999; 163(6):3250-9. (Clone-specific: Flow cytometry). View Reference
-
Macardle PJ, Chen Z, Shih CY, et al. Characterization of human leucocytes bearing the IL-3 receptor. Cell Immunol. 1996; 168(1):59-68. (Biology). View Reference
-
Miyajima A. CDw123 (Interleukin 3 receptor α chain) Workshop Panel report. In: Kishimoto T. Tadamitsu Kishimoto .. et al., ed. Leucocyte typing VI : white cell differentiation antigens : proceedings of the sixth international workshop and conference held in Kobe, Japan, 10-14 November 1996. New York: Garland Pub.; 1997:854-855.
-
Sun Q, Woodcock JM, Rapoport A, et al. Monoclonal antibody 7G3 recognizes the N-terminal domain of the human interleukin-3 (IL-3) receptor alpha-chain and functions as a specific IL-3 receptor antagonist.. Blood. 1996; 87(1):83-92. (Immunogen: Flow cytometry, Immunoprecipitation, Western blot). View Reference
-
Yamada T, Sun Q, Zeibecoglou K, et al. IL-3, IL-5, granulocyte-macrophage colony-stimulating factor receptor alpha-subunit, and common beta-subunit expression by peripheral leukocytes and blood dendritic cells.. J Allergy Clin Immunol. 1998; 101(5):677-82. (Clone-specific: Flow cytometry). View Reference
-
Zola H. Leukocyte and stromal cell molecules : the CD markers. Hoboken, N.J.: Wiley-Liss; 2007.
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.