-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- Denmark (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
Flow Cytometry–based Companion Diagnostics (CDx) Solutions
Built on a foundation of excellence and immunological expertise, BD Biosciences is committed to unlocking the potential of flow cytometry–based CDx solutions. With increasingly complex target and drug combinations, cutting-edge therapies require cutting-edge diagnostic solutions. Long established as the gold standard for immunophenotypic analysis1, flow cytometry provides the detailed proteomic analysis of multiple cell types, maximizing the amount of information gathered from rare cells2 that can influence critical decisions. From discovery to commercial launch, a collaborative partnership with BD provides the flexible, end-to-end solutions needed to rapidly advance your scientific breakthroughs.

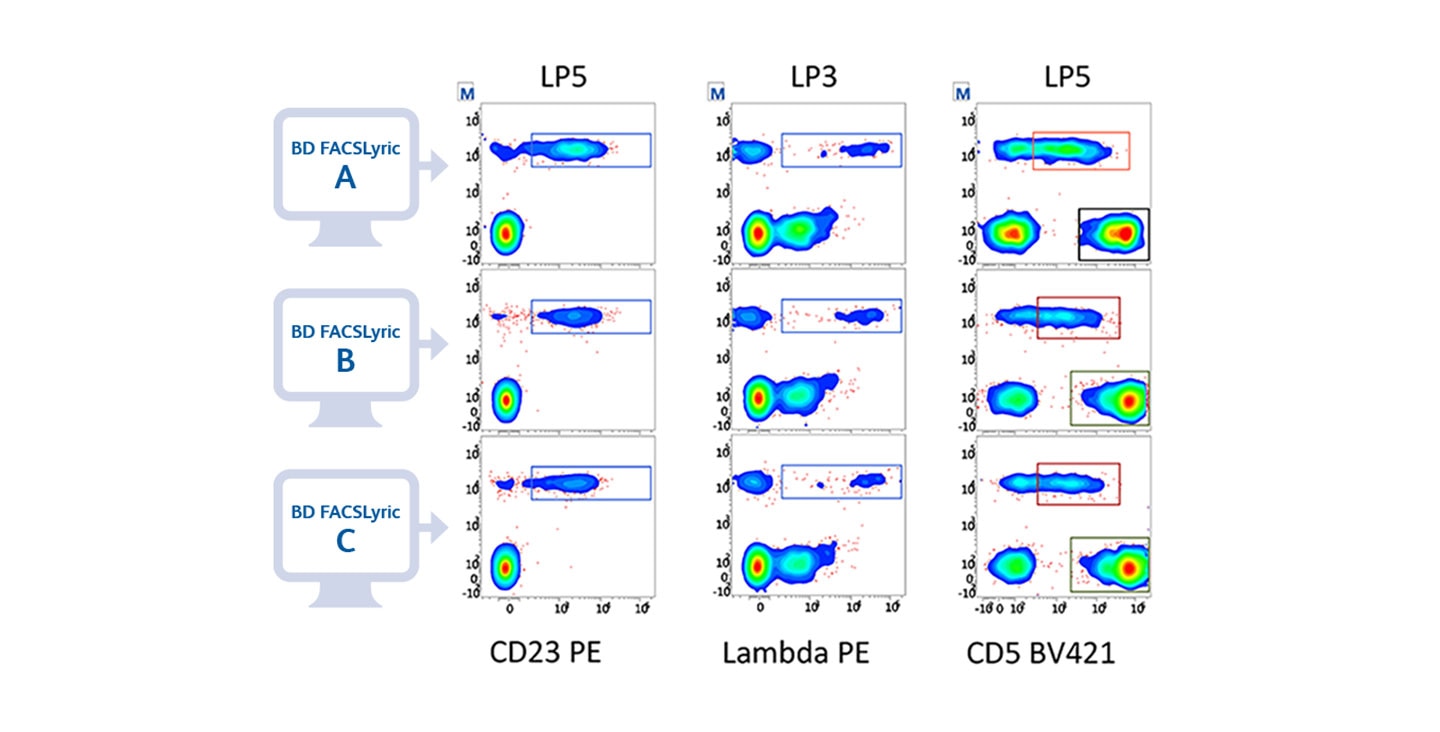
*High sensitivity and specificity demonstrated via IVD assays cleared on BD FACSLyric™ Flow Cytometer.
Workflow
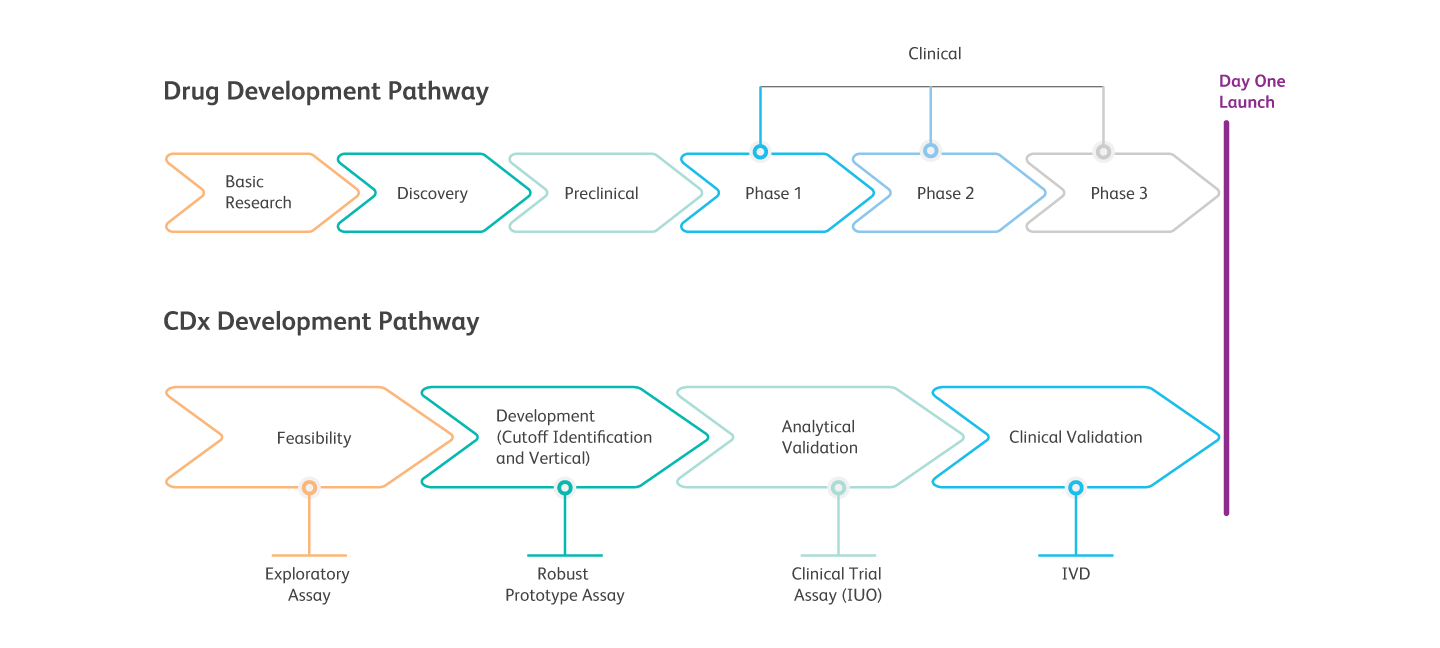
Comprehensive CDx Development Process
BD has an experienced team and leading flow cytometry technologies to support comprehensive CDx co-development from discovery to global commercialization. We offer the flexibility to collaborate at any stage of therapeutic, biomarker or diagnostic development.
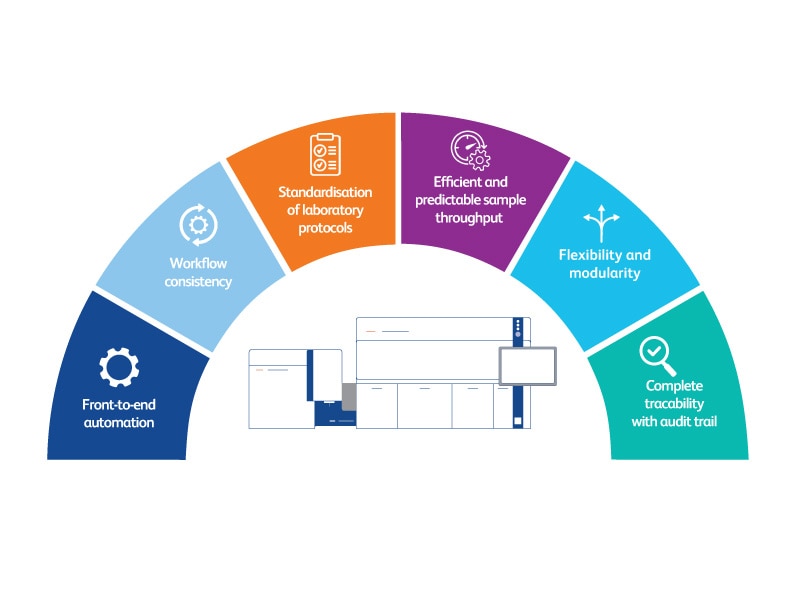
Built on a foundation of excellence, experience and expertise, the BD FACSLyric™ Flow Cytometry System (CE-IVD) provides the standardization and sensitivity necessary for next-generation clinical trial assays and companion diagnostics.
By combining automation, simplicity and speed, BD is creating a new, streamlined workflow to meet increasingly complex therapeutic options.
With U.S FDA 21 CFR Part 11–software features, cutting-edge reagents and unparalleled support, BD is the partner of choice for flow cytometric–based companion diagnostics.
The Power of the BD FACSLyric™ Flow Cytometer:
- 4-, 6-, 8-, 10- and 12-color configurations. Onsite upgradeable to adapt to your lab’s changing needs
- Up to 3 lasers—blue, red and violet—12 fluorescence channels and 14 parameters
- 35,000 events per second maximum acquisition rate; no limit on number of events acquired
- Automated single-tube QC with BD® CS&T Beads
- Fluorescence compensation required only every 60 days, improving efficiency and productivity
- 21 different loading options: 96 and 384 well plates or tubes; built-in flexibility with the BD FACS™ Universal Loader
- Powered by the BD FACSuite™ Acquisition and Analysis Application, whose password protection, audit trail, electronic signatures and IQ/OQ procedures assist in supporting U.S. FDA 21 CFR Part 11 features
- The BD FACS™ Workflow Manager Laboratory Information System (LIS) Interface and BD Assurity Linc™ Remote Systems Management Software streamline your workflow and help improve productivity through seamless integration
BD offers a comprehensive IVDR compliant portfolio including CE-IVD antibodies and reagent kit solutions for Oncohaematology and Immunology applications from our certified production sites. Recently the portfolio has been expanded with the CE-IVD MRD monitoring reagent solutions for standardised workflows from sample preparation to analysis for BCP-ALL and Multiple Myeloma.
In Vitro Diagnostic Medical Device Regulation
BD also offers custom and catalog panels in liquid, dried and lyophilized formats to minimize the errors and time associated with manual cocktailing of reagents. Dried and lyophilized formats increase reagent stability and significantly enhance consistency and assay performance. These offerings include contract manufacturing of pre-aliquoted, performance-optimized multicolor panels for flow cytometry in a dried down, ready-to-use format. The dried multicolor panels reduce inter-experiment variability and come with long shelf lives making them ideal choices for multisite and longitudinal studies.
| Liquid Cocktails | BD Horizon™ Dri Chroma | BD Horizon™ Lyo | |
|---|---|---|---|
| Product Offering | Liquid Cocktail | Preformulated Dried Cocktail | Preformulated Lyophilized/ Freeze-dried Cocktail |
| Capability | Standard dyes + up to 1 BD Horizon Brilliant™ Dye per vial | Standard dyes + up to 5 BD Horizon Brilliant™Dyes per tube | Standard dyes + up to 1 BD Horizon Brilliant™ Dye per tube |
| Packaging | Vials: 25–100 tests per vial | Tubes (no caps) | Tubes (with caps), plates, microtubes, multi-test vials |
| Stability | Stability varies based on cocktail composition | 12 months | 18 months |
| Min. Order Qty | 150 tests | 250 tests | <200 tests 20 plates (96-well plates) |
| Storage Conditions | 2–8 °C | 20–25 °C | 20–25 °C |
BD has an experienced team and the leading flow cytometry technologies to support comprehensive CDx co-development from discovery to global commercialization.
We offer the flexibility to engage with partners at any stage of therapeutic, biomarker or diagnostic development.
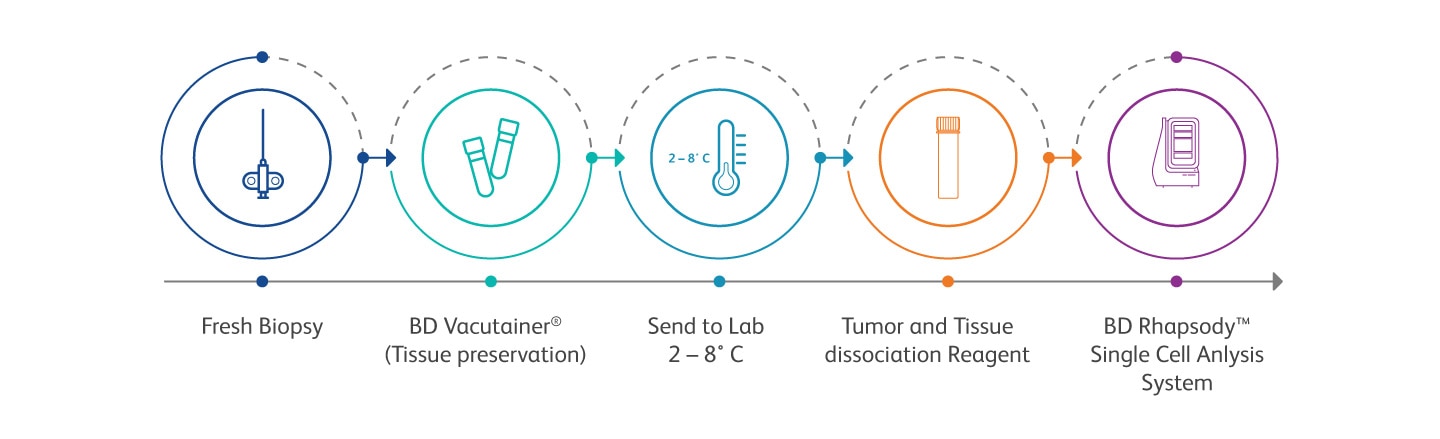
The BD Horizon™ Dri Tumor and Tissue Dissociation Reagent (TTDR) offers gentle and effective dissociation with superior epitope preservation. TTDR maximizes cell yields, while minimizing cell death, which allows effective dissociation of a variety of tumor types to enable single-cell studies.
Tumor types evaluated by BD or external investigators include lung3, colon3, and melanoma.
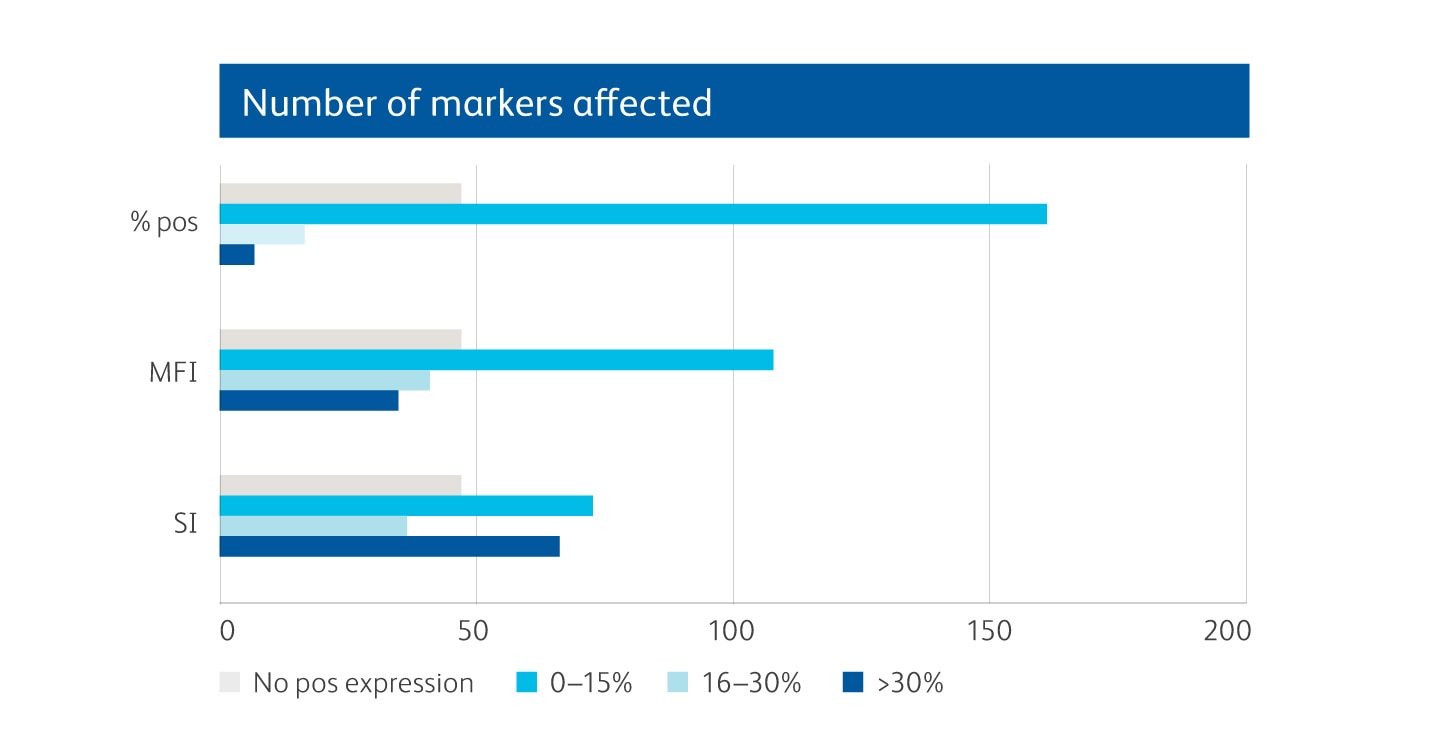
Graphical depiction demonstrating majority markers measured had preserved epitopes measured by MFI and SI, showing less than 0–30% change following dissociation.
Enabling analysis of solid tumor biopsies by flow cytometry
BD products have been used for analyzing solid tumor biopsies using flow cytometry.
See publications related to this workflow
The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies
STAR Protocol
Isolation of tumor-infiltrating lymphocytes from preserved human tumor tissue specimens for downstream characterization
BD Biosciences is an industry leader in flow cytometry with over 45 years of flow cytometry expertise and 20,000+ catalog reagents used in 190 countries across the globe. With over sixty million IVD tests sold worldwide, we have the global infrastructure and support teams needed to successfully create, develop and launch a companion diagnostic test. Our dedicated CDx team will work closely with you throughout the entire process to ensure a successful day one launch.
BD has extensive global reach and IVD regulatory experience to support your CDx program.
Built on a foundation of excellence, experience and expertise, the BD FACSLyric™ Flow Cytometry System (CE-IVD) provides the standardization and sensitivity necessary for next-generation clinical trial assays and companion diagnostics.
By combining automation, simplicity and speed, BD is creating a new, streamlined workflow to meet increasingly complex therapeutic options.
With U.S FDA 21 CFR Part 11–software features, cutting-edge reagents and unparalleled support, BD is the partner of choice for flow cytometric–based companion diagnostics.
The Power of the BD FACSLyric™ Flow Cytometer:
- 4-, 6-, 8-, 10- and 12-color configurations. Onsite upgradeable to adapt to your lab’s changing needs
- Up to 3 lasers—blue, red and violet—12 fluorescence channels and 14 parameters
- 35,000 events per second maximum acquisition rate; no limit on number of events acquired
- Automated single-tube QC with BD® CS&T Beads
- Fluorescence compensation required only every 60 days, improving efficiency and productivity
- 21 different loading options: 96 and 384 well plates or tubes; built-in flexibility with the BD FACS™ Universal Loader
- Powered by the BD FACSuite™ Acquisition and Analysis Application, whose password protection, audit trail, electronic signatures and IQ/OQ procedures assist in supporting U.S. FDA 21 CFR Part 11 features
- The BD FACS™ Workflow Manager Laboratory Information System (LIS) Interface and BD Assurity Linc™ Remote Systems Management Software streamline your workflow and help improve productivity through seamless integration
BD offers a comprehensive IVDR compliant portfolio including CE-IVD antibodies and reagent kit solutions for Oncohaematology and Immunology applications from our certified production sites. Recently the portfolio has been expanded with the CE-IVD MRD monitoring reagent solutions for standardised workflows from sample preparation to analysis for BCP-ALL and Multiple Myeloma.
In Vitro Diagnostic Medical Device Regulation
BD also offers custom and catalog panels in liquid, dried and lyophilized formats to minimize the errors and time associated with manual cocktailing of reagents. Dried and lyophilized formats increase reagent stability and significantly enhance consistency and assay performance. These offerings include contract manufacturing of pre-aliquoted, performance-optimized multicolor panels for flow cytometry in a dried down, ready-to-use format. The dried multicolor panels reduce inter-experiment variability and come with long shelf lives making them ideal choices for multisite and longitudinal studies.
| Liquid Cocktails | BD Horizon™ Dri Chroma | BD Horizon™ Lyo | |
|---|---|---|---|
| Product Offering | Liquid Cocktail | Preformulated Dried Cocktail | Preformulated Lyophilized/ Freeze-dried Cocktail |
| Capability | Standard dyes + up to 1 BD Horizon Brilliant™ Dye per vial | Standard dyes + up to 5 BD Horizon Brilliant™Dyes per tube | Standard dyes + up to 1 BD Horizon Brilliant™ Dye per tube |
| Packaging | Vials: 25–100 tests per vial | Tubes (no caps) | Tubes (with caps), plates, microtubes, multi-test vials |
| Stability | Stability varies based on cocktail composition | 12 months | 18 months |
| Min. Order Qty | 150 tests | 250 tests | <200 tests 20 plates (96-well plates) |
| Storage Conditions | 2–8 °C | 20–25 °C | 20–25 °C |
BD has an experienced team and the leading flow cytometry technologies to support comprehensive CDx co-development from discovery to global commercialization.
We offer the flexibility to engage with partners at any stage of therapeutic, biomarker or diagnostic development.
The BD Horizon™ Dri Tumor and Tissue Dissociation Reagent (TTDR) offers gentle and effective dissociation with superior epitope preservation. TTDR maximizes cell yields, while minimizing cell death, which allows effective dissociation of a variety of tumor types to enable single-cell studies.
Tumor types evaluated by BD or external investigators include lung3, colon3, and melanoma.

Graphical depiction demonstrating majority markers measured had preserved epitopes measured by MFI and SI, showing less than 0–30% change following dissociation.
Enabling analysis of solid tumor biopsies by flow cytometry
BD products have been used for analyzing solid tumor biopsies using flow cytometry.
See publications related to this workflow
The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies
STAR Protocol
Isolation of tumor-infiltrating lymphocytes from preserved human tumor tissue specimens for downstream characterization

BD Biosciences is an industry leader in flow cytometry with over 45 years of flow cytometry expertise and 20,000+ catalog reagents used in 190 countries across the globe. With over sixty million IVD tests sold worldwide, we have the global infrastructure and support teams needed to successfully create, develop and launch a companion diagnostic test. Our dedicated CDx team will work closely with you throughout the entire process to ensure a successful day one launch.
BD has extensive global reach and IVD regulatory experience to support your CDx program.

Why BD?
BD is your dependable partner for CDx
BD is an excellent partner for CDx development based on acknowledged diagnostic expertise:
- Deep commercial experience with flow cytometry
- Dedicated CDx team
- Global install base
- Regulatory compliance (IVD/IVDR)
- End-to-end solutions
Global Diagnostics Expertise and Footprint

Contact Us
Our dedicated CDx team provides solutions ranging from assay development to CDx commercialization. If you have any questions or would like to learn more about how BD can help, please provide your contact information and someone from our team will personally reach out to you.
*Required fields
-
Brochure
References
- Rane AS, Rutkauskaite J, DeMello A, Stavrakis S. High-throughput multi-parametric imaging flow cytometry. Chem. 2017. 3(4):588-602.
- Roshal M, Flores-Montero JA, Gao Q, Koeber M, Wardrope J. et al. MRD detection in multiple myeloma: comparison between MSKCC 10-color single-tube and EuroFlow 8-color 2-tube methods. Blood Adv. 2017. 1(12):728-732. https://doi.org/10.1182/bloodadvances.2016003715
- Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of Pd-1 blockade therapies. Nature Immunology. 2020. 21, 1346-1358
The BD FACSLyric™ Flow Cytometer and BD FACSDuet™ Sample Preparation System are Class 1 Laser Products.
![]()
BD FACSLyric™ Flow Cytometer with the BD FACSuite™ Clinical and BD FACSuite™ Applications is an in vitro diagnostic medical device bearing a CE mark. The BD FACSDuet™ Sample Preparation System is an in vitro diagnostic medical device bearing a CE marked. Sample preparation for user-defined protocols and cocktailing functions have not been validated for IVD use and require validation by the user.
Other BD products included in this document are for Research Use Only. Not for use in diagnostic or therapeutic procedures.