-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- BD OMICS-One™ WTA Next Assay
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?

Broaden Your Lab’s Potential
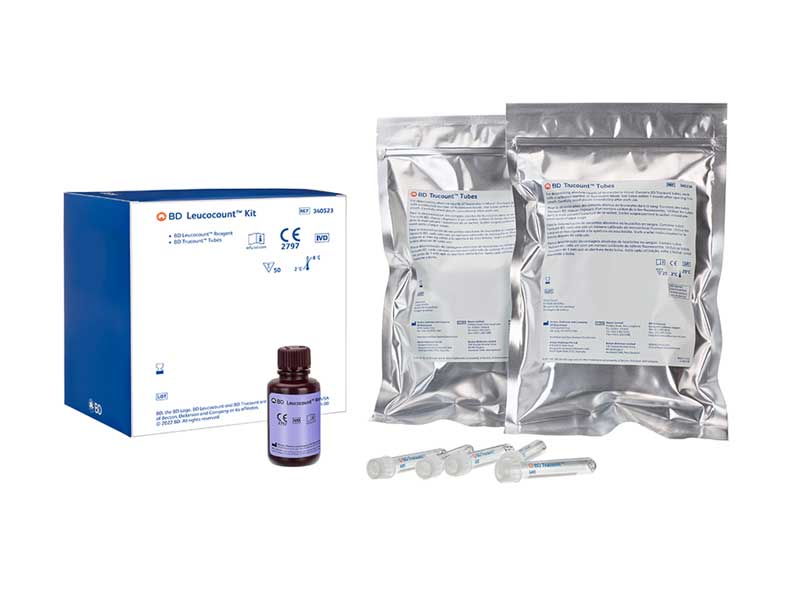
Access the proven BD Leucocount™ Kit, now on the BD FACSLyric™ Flow Cytometer
Overview
Have full confidence in your residual white blood cell enumeration by adding the BD Leucocount™ Kit to the expanding menu of BD CE-IVD assays on the BD FACSLyric™ Flow Cytometer. This automated solution allows you to not only deliver sensitivity that aligns with leucoreduction guidelines but also streamline your workflow. Advance your capabilities with quality control you can rely on for safe blood transfusion products.
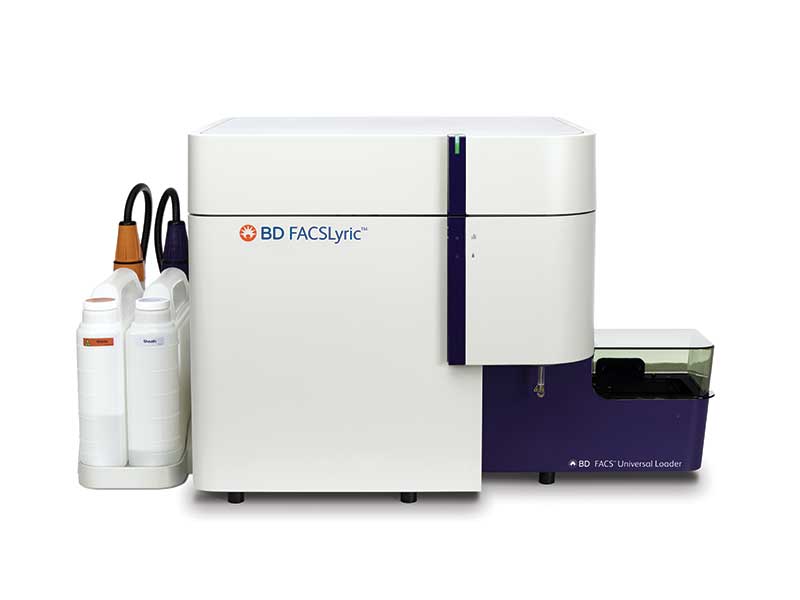
BD Leucocount™ Kit now on the BD FACSLyric™ Flow Cytometer
- Achieve reliable enumeration of residual white blood cells with a standardized CE-IVD assay
- Assess safety of blood transfusion products with sensitivity that aligns with leucoreduction guidelines
- Enable high-throughput capability delivering results that seamlessly transfer through LIS integration
![]()
The BD FACSLyric™ Flow Cytometer is a Class 1 Laser Product.
BD FACSLyric™ Flow Cytometer with the BD FACS™ Universal Loader, BD FACSuite™ Clinical and BD FACSuite™ Applications is an in vitro diagnostic medical device bearing a CE mark.
BD Leucocount™ Kit and BD Leucocount™ RBC and PLT Controls are in vitro diagnostic medical devices bearing a CE mark and are CE certified by BSI Group the Netherlands B.V. (Notified Body Number = 2797).