-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- Switzerland (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
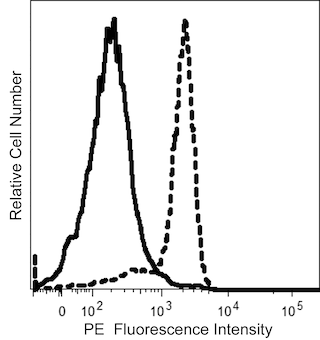
BD™ AbSeq Oligo Mouse Anti-Human GITR (CD357)
Clone V27-580 (RUO)


Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
Put all BD® AbSeq Reagents to be pooled into a Latch Rack for 500 µL Tubes (Thermo Fisher Scientific Cat. No. 4900). Arrange the tubes so that they can be easily uncapped and re-capped with an 8-Channel Screw Cap Tube Capper (Thermo Fisher Scientific Cat. No. 4105MAT) and the reagents aliquoted with a multi-channel pipette.
BD® AbSeq tubes should be centrifuged for ≥ 30 seconds at 400 × g to ensure removal of any content in the cap/tube threads prior to the first opening.
Product Notices
- This reagent has been pre-diluted for use at the recommended volume per test. Typical use is 2 µl for 1 × 10^6 cells in a 200-µl staining reaction.
- The production process underwent stringent testing and validation to assure that it generates a high-quality conjugate with consistent performance and specific binding activity. However, verification testing has not been performed on all conjugate lots.
- Please refer to bd.com/genomics-resources for technical protocols.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Illumina is a trademark of Illumina, Inc.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- For U.S. patents that may apply, see bd.com/patents.
Data Sheets
Companion Products



Recently Viewed
The V27-580 monoclonal antibody specifically binds to GITR (Glucocorticoid-Induced Tumor necrosis factor Receptor), a member of the tumor necrosis factor receptor (TNFR) superfamily that is designated TNFRSF18. In the human, GITR is expressed at low levels in peripheral blood T cells, bone marrow, thymus, spleen, and lymph nodes and is up-regulated upon antigen stimulation or by treatment with anti-CD3 plus anti-CD28. GITR is also reported to be constitutively expressed on Treg cells. GITR's ligand (GITRL) is a member of the TNF superfamily, is designated TNFSF18, and is expressed on antigen presenting cells. The GITR cytoplasmic domain has striking homology with the cytoplasmic domains of the co-stimulatory receptors CD137 (4-1BB), CD134 (OX40) and CD27. GITR signaling is mediated by signaling adaptors, TNFR-associated factors (TRAFs), that affect signaling pathways (eg, Erk, JNK, MAPK and NF-κB) to enhance T-cell survival and cytokine production. The effects of GITR signaling upon the dynamic and interconnected roles of effector and regulatory T lymphocytes in the immune response are under investigation.
Development References (7)
-
Clouthier DL, Watts TH. Cell-specific and context-dependent effects of GITR in cancer, autoimmunity, and infection.. Cytokine Growth Factor Rev. 2014; 25(2):91-106. (Biology). View Reference
-
Kwon B, Yu KY, Ni J, et al. Identification of a novel activation-inducible protein of the tumor necrosis factor receptor superfamily and its ligand.. J Biol Chem. 1999; 274(10):6056-61. (Biology). View Reference
-
Li Z, Mahesh SP, Kim BJ, Buggage RR, Nussenblatt RB. Expression of glucocorticoid induced TNF receptor family related protein (GITR) on peripheral T cells from normal human donors and patients with non-infectious uveitis.. J Autoimmun. 2003; 21(1):83-92. (Biology). View Reference
-
Nocentini G, Giunchi L, Ronchetti S, et al. A new member of the tumor necrosis factor / nerve growth factor receptor family inhibits T cell receptor-induced apoptosis... Proc Natl Acad Sci U S A. 1997; 94(12):6216-6221. (Biology). View Reference
-
Pedroza-Gonzalez A, Zhou G, Singh SP, et al. GITR engagement in combination with CTLA-4 blockade completely abrogates immunosuppression mediated by human liver tumor-derived regulatory T cells ex vivo.. Oncoimmunology. 2015; 4(12):e1051297. (Biology). View Reference
-
Placke T, Kopp HG, Salih HR. Glucocorticoid-induced TNFR-related (GITR) protein and its ligand in antitumor immunity: functional role and therapeutic modulation.. Clin Dev Immunol. 2010; 2010:239083. (Biology). View Reference
-
Shevach EM, Stephens GL. The GITR-GITRL interaction: co-stimulation or contrasuppression of regulatory activity?. Nat Rev Immunol. 2006; 6(8):613-8. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.

