-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- Austria (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
BD Pharmingen™ Purified NA/LE Anti-Human CD20 Obinutuzumab Biosimilar
Clone Obinutuzumab.rMAb (also known as Obinutuzumab Biosimilar) (RUO)
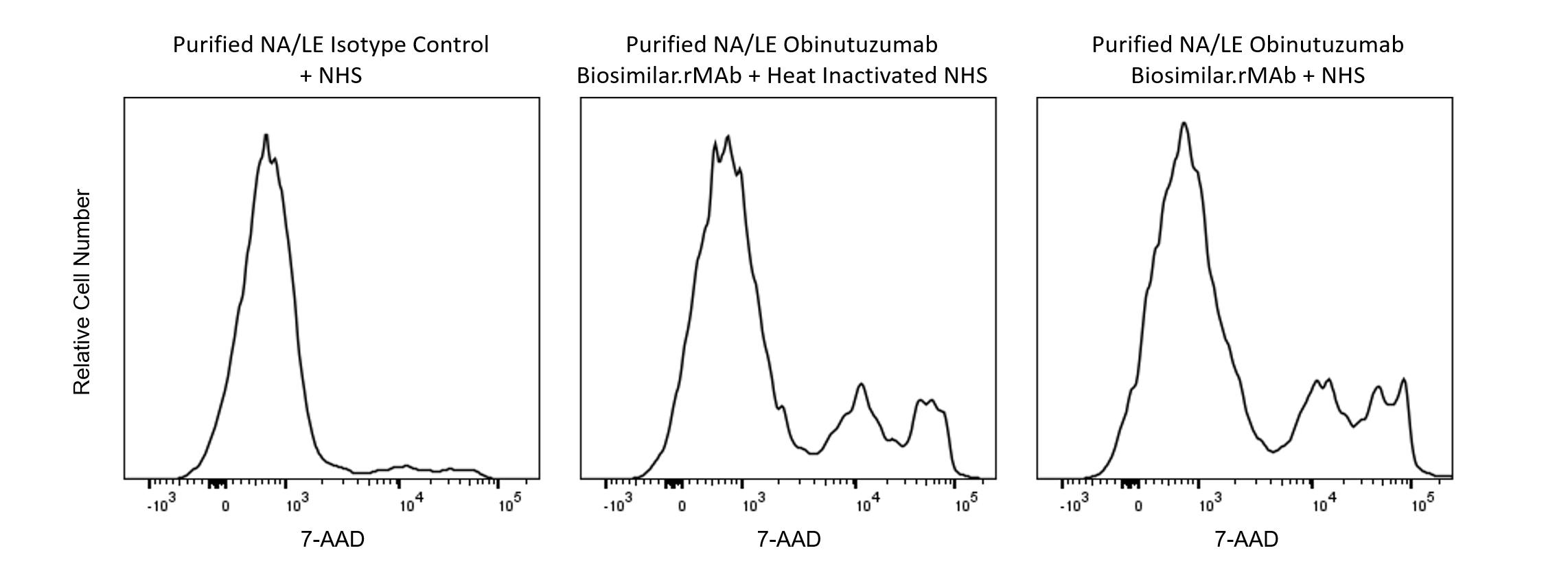
Flow cytometric analysis of Human Daudi cells after treatment using the cytotoxic Obinutuzumab.rMAb in the presence of Normal Human Serum (NHS) or heat-inactivated (complement-inactivated) NHS. Cells from the Human Daudi (Burkitt's lymphoma, ATCC® CRL-213™) cell line (1 x 10^6 cells/ml) were maintained in RPMI media containing heat-inactivated fetal bovine serum (56°C for 30 min to remove complement activity). The cells were treated with 1.25 µg/ml of either Purified NA/LE Human IgG1, κ Isotype Control (Cat. No. 569605) or Purified NA/LE Anti-Human CD20 Obinutuzumab Biosimilar (Cat. No. 570921) followed by addition of a 25% final concentration of NHS (as a source of complement) or heat-inactivated NHS (complement-inactivated) as indicated and incubated at 37°C for 5 hours. The cells were then harvested and resuspended in BD Pharmingen™ Stain Buffer (FBS) [Cat. No. 554656]. BD Via-Probe™ Cell Viability 7-AAD Solution (Cat. No. 555815) was added to cells right before flow cytometric analysis to distinguish viable (7-AAD-) and nonviable (7-AAD+) cells based on the histograms showing 7-AAD fluorescence. Flow cytometry and data analysis were performed using a BD LSRFortessa™ X-20 Cell Analyzer System and FlowJo™ software. Data shown on this Technical Data Sheet are not lot specific.
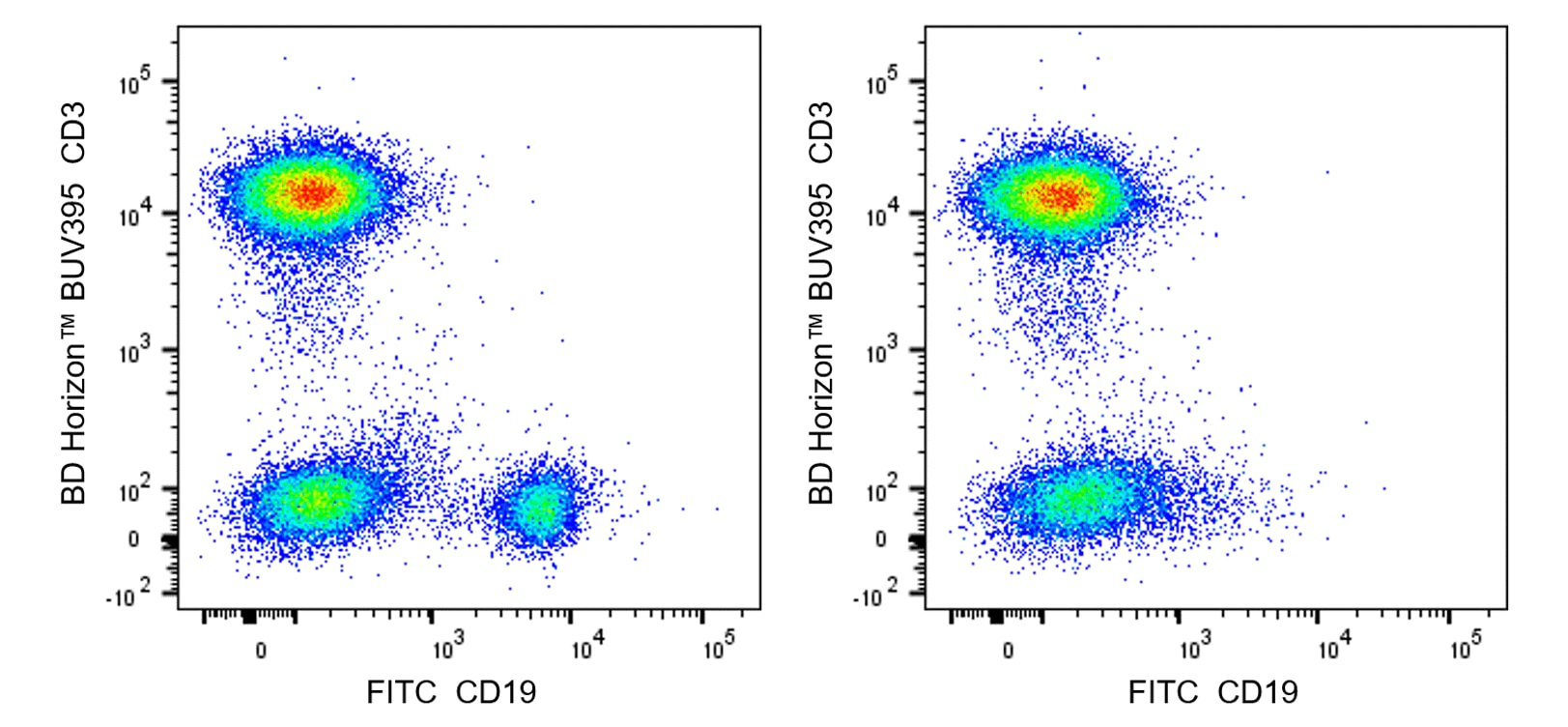
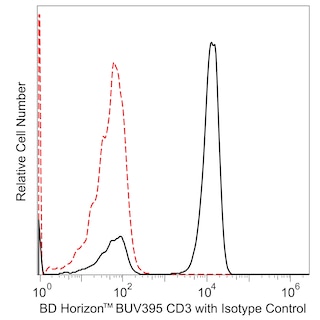
Multicolor flow cytometric analysis of viable Human peripheral blood lymphocytes after treatment of PBMC with the cytotoxic Obinutuzumab.rMAb in the presence of complement-inactivated NHS. Human peripheral blood mononuclear cells (PBMC) were maintained in RPMI media containing heat-inactivated fetal bovine serum (56°C for 30 min to remove complement activity). Purified NA/LE Human IgG1, κ Isotype Control (Cat. No. 569605; Left Plot) or Purified NA/LE Anti-Human CD20 Obinutuzumab Biosimilar (Cat. No. 570921; Right Plot) were added to the PBMC that were cultured with heat-inactivated NHS (37°C for 2 hours). The cells were harvested and stained with FITC Mouse Anti-Human CD19 (Cat. No. 555412) and BD Horizon™ BUV395 Mouse Anti-Human CD3 (Cat. No. 563546) antibodies. BD Via-Probe™ Cell Viability 7-AAD Solution (Cat. No. 555815) was added to the cells right before analysis. The bivariate pseudocolor density plots showing the correlated expression of CD19 versus CD3 were derived from gated events with the forward and side light-scatter characteristics of viable (7-AAD-negative) lymphocyte populations. Flow cytometric analysis was performed using a BD Fortessa™ X-20 Flow Cytometer System and FlowJo™ software.



Flow cytometric analysis of Human Daudi cells after treatment using the cytotoxic Obinutuzumab.rMAb in the presence of Normal Human Serum (NHS) or heat-inactivated (complement-inactivated) NHS. Cells from the Human Daudi (Burkitt's lymphoma, ATCC® CRL-213™) cell line (1 x 10^6 cells/ml) were maintained in RPMI media containing heat-inactivated fetal bovine serum (56°C for 30 min to remove complement activity). The cells were treated with 1.25 µg/ml of either Purified NA/LE Human IgG1, κ Isotype Control (Cat. No. 569605) or Purified NA/LE Anti-Human CD20 Obinutuzumab Biosimilar (Cat. No. 570921) followed by addition of a 25% final concentration of NHS (as a source of complement) or heat-inactivated NHS (complement-inactivated) as indicated and incubated at 37°C for 5 hours. The cells were then harvested and resuspended in BD Pharmingen™ Stain Buffer (FBS) [Cat. No. 554656]. BD Via-Probe™ Cell Viability 7-AAD Solution (Cat. No. 555815) was added to cells right before flow cytometric analysis to distinguish viable (7-AAD-) and nonviable (7-AAD+) cells based on the histograms showing 7-AAD fluorescence. Flow cytometry and data analysis were performed using a BD LSRFortessa™ X-20 Cell Analyzer System and FlowJo™ software. Data shown on this Technical Data Sheet are not lot specific.
Multicolor flow cytometric analysis of viable Human peripheral blood lymphocytes after treatment of PBMC with the cytotoxic Obinutuzumab.rMAb in the presence of complement-inactivated NHS. Human peripheral blood mononuclear cells (PBMC) were maintained in RPMI media containing heat-inactivated fetal bovine serum (56°C for 30 min to remove complement activity). Purified NA/LE Human IgG1, κ Isotype Control (Cat. No. 569605; Left Plot) or Purified NA/LE Anti-Human CD20 Obinutuzumab Biosimilar (Cat. No. 570921; Right Plot) were added to the PBMC that were cultured with heat-inactivated NHS (37°C for 2 hours). The cells were harvested and stained with FITC Mouse Anti-Human CD19 (Cat. No. 555412) and BD Horizon™ BUV395 Mouse Anti-Human CD3 (Cat. No. 563546) antibodies. BD Via-Probe™ Cell Viability 7-AAD Solution (Cat. No. 555815) was added to the cells right before analysis. The bivariate pseudocolor density plots showing the correlated expression of CD19 versus CD3 were derived from gated events with the forward and side light-scatter characteristics of viable (7-AAD-negative) lymphocyte populations. Flow cytometric analysis was performed using a BD Fortessa™ X-20 Flow Cytometer System and FlowJo™ software.

Flow cytometric analysis of Human Daudi cells after treatment using the cytotoxic Obinutuzumab.rMAb in the presence of Normal Human Serum (NHS) or heat-inactivated (complement-inactivated) NHS. Cells from the Human Daudi (Burkitt's lymphoma, ATCC® CRL-213™) cell line (1 x 10^6 cells/ml) were maintained in RPMI media containing heat-inactivated fetal bovine serum (56°C for 30 min to remove complement activity). The cells were treated with 1.25 µg/ml of either Purified NA/LE Human IgG1, κ Isotype Control (Cat. No. 569605) or Purified NA/LE Anti-Human CD20 Obinutuzumab Biosimilar (Cat. No. 570921) followed by addition of a 25% final concentration of NHS (as a source of complement) or heat-inactivated NHS (complement-inactivated) as indicated and incubated at 37°C for 5 hours. The cells were then harvested and resuspended in BD Pharmingen™ Stain Buffer (FBS) [Cat. No. 554656]. BD Via-Probe™ Cell Viability 7-AAD Solution (Cat. No. 555815) was added to cells right before flow cytometric analysis to distinguish viable (7-AAD-) and nonviable (7-AAD+) cells based on the histograms showing 7-AAD fluorescence. Flow cytometry and data analysis were performed using a BD LSRFortessa™ X-20 Cell Analyzer System and FlowJo™ software. Data shown on this Technical Data Sheet are not lot specific.

Multicolor flow cytometric analysis of viable Human peripheral blood lymphocytes after treatment of PBMC with the cytotoxic Obinutuzumab.rMAb in the presence of complement-inactivated NHS. Human peripheral blood mononuclear cells (PBMC) were maintained in RPMI media containing heat-inactivated fetal bovine serum (56°C for 30 min to remove complement activity). Purified NA/LE Human IgG1, κ Isotype Control (Cat. No. 569605; Left Plot) or Purified NA/LE Anti-Human CD20 Obinutuzumab Biosimilar (Cat. No. 570921; Right Plot) were added to the PBMC that were cultured with heat-inactivated NHS (37°C for 2 hours). The cells were harvested and stained with FITC Mouse Anti-Human CD19 (Cat. No. 555412) and BD Horizon™ BUV395 Mouse Anti-Human CD3 (Cat. No. 563546) antibodies. BD Via-Probe™ Cell Viability 7-AAD Solution (Cat. No. 555815) was added to the cells right before analysis. The bivariate pseudocolor density plots showing the correlated expression of CD19 versus CD3 were derived from gated events with the forward and side light-scatter characteristics of viable (7-AAD-negative) lymphocyte populations. Flow cytometric analysis was performed using a BD Fortessa™ X-20 Flow Cytometer System and FlowJo™ software.


BD Pharmingen™ Purified NA/LE Anti-Human CD20 Obinutuzumab Biosimilar

BD Pharmingen™ Purified NA/LE Anti-Human CD20 Obinutuzumab Biosimilar

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Product Notices
- For U.S. patents that may apply, see bd.com/patents.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- An isotype control should be used at the same concentration as the antibody of interest.
Data Sheets
Companion Products



The Obinutuzumab.rMAb (also known as, Obinutuzumab Biosimilar) is a research grade humanized recombinant human IgG1, kappa antibody that specifically recognizes the extracellular domain of human CD20 similarly to the therapeutic Obinutuzumab antibody. The Obinutuzumab.rMAb uses the same variable region sequences as Obinutuzumab, combined with constant region sequences derived from consensus sequences of human IgG1, kappa. Obinutuzumab triggers target cell death through multiple mechanisms including complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), and apoptosis. The therapeutic Obinutuzumab has been approved to treat human B cell malignancies, autoimmune disorders, and transplant rejection. CD20 is a 33-37 kDa, unglycosylated four-transmembrane phosphoprotein with a cytoplasmic N-terminus and C-terminus that is encoded by MS4A1 (Membrane-spanning 4-domains, subfamily A, member 1). CD20 is expressed on pre-B-cells, naive and activated B cells, memory B cells, and at lower levels on a small subset of T cells but is absent from plasmablasts and plasma cells. It is also expressed on most malignant B cells and CD20-positive T-cell lymphomas. CD20 functions in mediating calcium transport and B cell activation, differentiation, and survival.
The Obinutuzumab.rMAb is intended for research use only. It is not intended for use in therapeutic or diagnostic procedures for humans or animals.
Development References (4)
-
Almasri NM, Duque RE, Iturraspe J, Everett E, Braylan RC. Reduced expression of CD20 antigen as a characteristic marker for chronic lymphocytic leukemia. Am J Hematol. 1992; 40:259-263. (Biology).
-
Brekke OH, Sandlie I. Therapeutic antibodies for human diseases at the dawn of the twenty-first century.. Nat Rev Drug Discov. 2003; 2(1):52-62. (Biology). View Reference
-
Hultin LE, Hausner MA, Hultin PM, Giorgi JV. CD20 (pan-B cell) antigen is expressed at a low level on asubpopulation of human T lymphocytes. Cytometry. 1993; 14:196-204. (Biology).
-
Tobinai K, Klein C, Oya N, Fingerle-Rowson G. A Review of Obinutuzumab (GA101), a Novel Type II Anti-CD20 Monoclonal Antibody, for the Treatment of Patients with B-Cell Malignancies.. Adv Ther. 2017; 34(2):324-356. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.