-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- BD OMICS-One™ WTA Next Assay
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?
BD FACSDuet™ Sample Preparation System
Overview
The BD FACSDuet™ sample preparation system provides a powerful new level of performance to move the pace of your lab forward. With pre-analytical automation with standardisation, the BD FACSDuet™ drives consistency and workflow efficiency. The BD FACSDuet™ also allows for automated cocktail preparation, greatly enhancing the flexibility and standardization. Designed to complement and physically integrate with the BD FACSLyric™ flow cytometer, the BD FACSDuet™ provides a complete walkaway sample-to-answer solution. Through its automation protocol, the BD FACSDuet™ enables higher lab productivity and accuracy in results by minimising manual intervention. The complete workflow traceability provided by the BD FACSDuet™ enables the lab to be compliant with ISO-15189 standards.

Features
Specimen (loading)
Delivering Flexibility in Specimen and Tube Type

The BD FACSDuet™ answers the growing needs of the laboratories to handle different types and sizes of specimen tubes and supports the need for consolidation and testing centralisation in hospitals. The BD FACSDuet™ delivers the flexibility that is required by supporting twenty-two (22) different types of blood collection tubes from different manufacturers. The choice includes BD Vacutainer®, Sarstedt S-Monovette™, Greiner and Streck tubes.
Specimen management is driven by tube adaptors. Seven (7) tube adaptors are available; they are barcoded and color coded for easy identification; they guarantee accurate tube piercing and sample volume dispensing.

Driving Workflow Efficiency and Throughput
Up to 40 specimen tubes can be accommodated at any given time in 4 racks holding 10 specimens each, driving workflow efficiency and throughput.

Two type of specimen racks are available, of which one is specifically designed for pediatric tubes (B)
Specimen traceability and automated worklist creation are guaranteed by 5 different barcodes on the specimen rack, identifying:
- Rack
- Tube position
- Empty position
- Adaptor
- Specimen
Automated barcode scanning during rack insertion enables:
- Patient specimen traceability
- Pipetting management
- Retrieving patient and assay information from Laboratory Information System (LIS) for automated worklist creation
Carrier (loading)
Allowing for Throughput and Traceability of the Samples and Worklist

Worklist traceability is enabled by the scanning of the carrier barcode that is associated with a specific worklist and defined specimens.
Secondary tubes are loaded in the BD FACSDuet™ onto 30 and/or 40 tube sample carriers, compatible with the BD FACSLyric™ loader and in the footprint of 96 well plate.
Barcoded secondary tubes are scanned to guarantee sample traceability and association to the specimen and the worklist
Reagents (loading)
Delivering Reagent Management and Flexibility in Panel Design

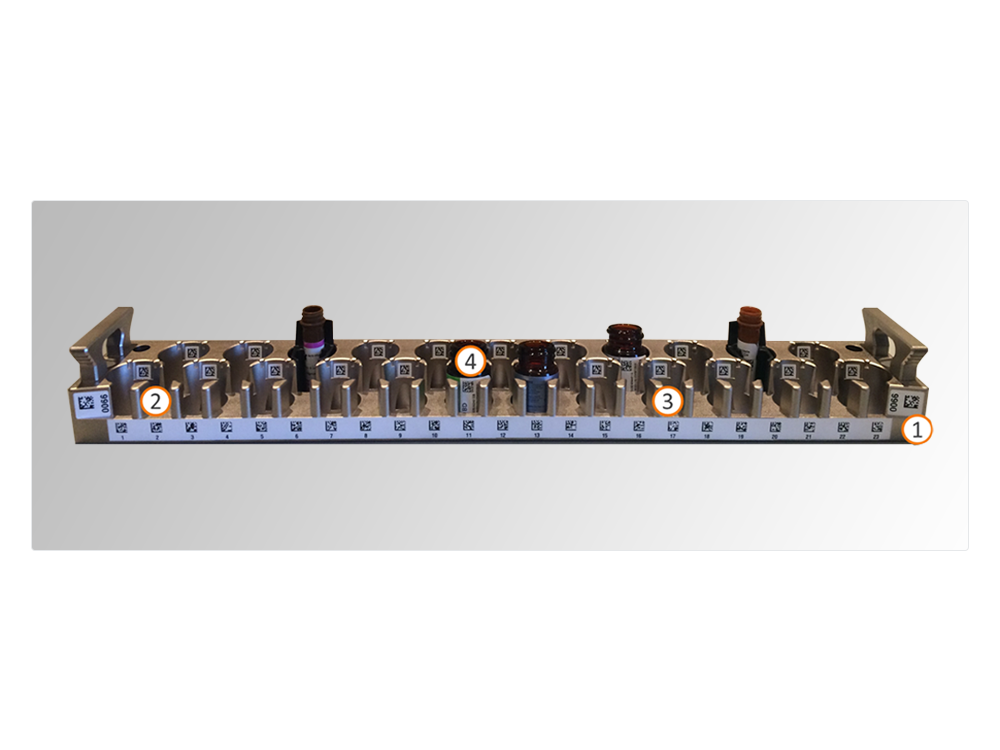
By accommodating up to 46 reagent vials in 2 separate racks, the BD FACSDuet™ delivers flexibility in antibody panel design and walkaway capabilities. Vial adaptors allow the use of reagent vials of different sizes.
In addition to the one on the antibody vial (4), the reagent rack carries 3 barcodes: one identifying the rack (1), another the reagent position (2) and a third identifying the empty position (3).
Barcodes on reagent racks and antibody vials drive reagent traceability, efficient antibody management and reduction of reagent waste.
Reagent (cocktailing)
The cocktailing functionality allows on-board, automated antibody cocktail preparation, eliminating the risk of errors due to manual pipetting and enabling the automated recording of all used antibodies for easier tracking and auditing. Part number, lot number and expiry date of the cocktail – as defined by the user – are also tracked and recorded to ensure quality and consistency in laboratory workflow.
Each antibody cocktail can be made from a maximum of 45 unique conjugates in a single cocktailing vial. Users’ cocktail recipes are stored in the software and available for subsequent preparations of the same cocktail. Once prepared, the cocktail is considered as any other reagent and available in the user reagent library for selection.
Cocktails designed to contain more than one BD Horizon™ Brilliant dye can include automated addition of BD Horizon™ Brilliant Stain Buffer or BD Horizon™ Brilliant Stain Buffer Plus, ensuring dye stability and staining performance.
The cocktailing software is an integral part of the BD FACSDuet software, and is as intuitive and easy-to-use as the rest of the software.
“What is the potential impact of the BD FACSDuet? It removes our manual processes, increases our efficiency and throughput, and gives us control in standardization of processes. It’s a controlled environment”
“What is the potential impact of the BD FACSDuet? It removes our manual processes, increases our efficiency and throughput, and gives us control in standardization of processes. It’s a controlled environment”
“I require all the monoclonal antibodies used, the ancillary reagents, tubes, the QC materials to be linked to an exact patient…this is an essential laboratory process and it is also mandatory in order to maintain ISO 15189 accreditation"

“I require all the monoclonal antibodies used, the ancillary reagents, tubes, the QC materials to be linked to an exact patient…this is an essential laboratory process and it is also mandatory in order to maintain ISO 15189 accreditation"


-
Brochure
-
Technical Specifications
-
Product List
-
Data
-
ESCCA Reports
BD FACSDuet™ is CE marked in compliance with the European In Vitro Diagnostic Medical Device Directive 98/79/EC.