Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
For optimal and reproducible results, BD Horizon Brilliant Stain Buffer should be used anytime two or more BD Horizon Brilliant dyes (including BD OptiBuild Brilliant reagents) are used in the same experiment. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. The BD Horizon Brilliant Stain Buffer was designed to minimize these interactions. More information can be found in the Technical Data Sheet of the BD Horizon Brilliant Stain Buffer (Cat. No. 563794).
Product Notices
- This antibody was developed for use in flow cytometry.
- The production process underwent stringent testing and validation to assure that it generates a high-quality conjugate with consistent performance and specific binding activity. However, verification testing has not been performed on all conjugate lots.
- Researchers should determine the optimal concentration of this reagent for their individual applications.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- BD Horizon Brilliant Stain Buffer is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,575,303; 8,354,239.
- BD Horizon Brilliant Violet 605 is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,227,187; 8,455,613; 8,575,303; 8,354,239.
Companion Products
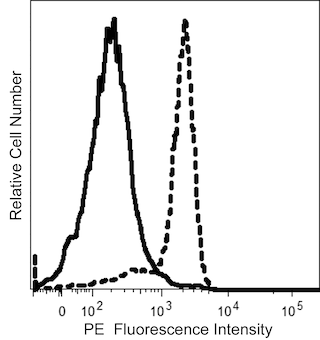




The 15D3 monoclonal antibody specifically recognizes P-glycoprotein which is also known as CD243, ATP-binding cassette subfamily B member 1 (ABCB1) or Multidrug resistance protein 1 (MDR1). P-glycoprotein is a ~170 kDa transmembrane glycoprotein protein that spans the membrane 12 times. P-glycoprotein acts as an ATP-dependent efflux pump for a large variety of drugs. It may be expressed at high levels by multidrug resistant (MDR) tumor cells. This efflux activity may lead to cellular resistance to the drugs used in chemotherapy. P-glycoprotein is present in many normal cell types including endothelial, epithelial, or secretory cells, and might protect them from naturally occurring xenobiotics.
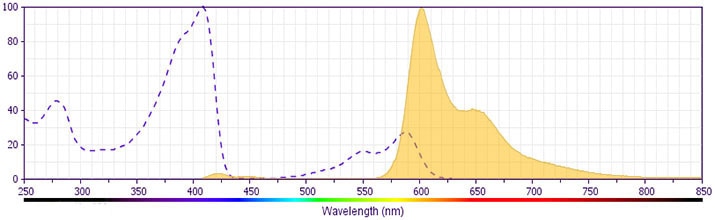
This antibody is conjugated to BD Horizon™ BV605 which is part of the BD Horizon Brilliant™ Violet family of dyes. With an Ex Max of 407-nm and Em Max of 602-nm, BD Horizon BV605 can be excited by a violet laser and detected with a standard 610/20-nm filter set. BD Horizon BV605 is a tandem fluorochrome of BD Horizon BV421 and an acceptor dye with an Em max at 605-nm. Due to the excitation of the acceptor dye by the green (532 nm) and yellow-green (561 nm) lasers, there will be significant spillover into the PE and BD Horizon PE-CF594 detectors off the green or yellow-green lasers. BD Horizon BV605 conjugates are very bright, often exhibiting brightness equivalent to PE conjugates and can be used as a third color off of the violet laser.
Development References (9)
-
Beck WT, Grogan TM, Willman CL, et al. Methods to detect P-glycoprotein-associated multidrug resistance in patients' tumors: consensus recommendations.. Cancer Res. 1996; 56(13):3010-20. (Biology). View Reference
-
Chan HS, Haddad G, Zheng L, Bradley G, Dalton WS, Ling V. Sensitive immunofluorescence detection of the expression of P-glycoprotein in malignant cells.. Cytometry. 1997; 29(1):65-75. (Biology). View Reference
-
Chaudhary PM, Mechetner EB, Roninson IB. Expression and activity of the multidrug resistance P-glycoprotein in human peripheral blood lymphocytes.. Blood. 1992; 80(11):2735-9. (Biology). View Reference
-
Dalton WS, Durie BG, Alberts DS, Gerlach JH, Cress AE. Characterization of a new drug-resistant human myeloma cell line that expresses P-glycoprotein.. Cancer Res. 1986; 46(10):5125-30. (Biology). View Reference
-
Drach D, Zhao S, Drach J, et al. Subpopulations of normal peripheral blood and bone marrow cells express a functional multidrug resistant phenotype.. Blood. 1992; 80(11):2729-34. (Biology). View Reference
-
Filipits M, Suchomel RW, Dekan G, et al. MRP and MDR1 gene expression in primary breast carcinomas.. Clin Cancer Res. 1996; 2(7):1231-7. (Biology). View Reference
-
Klimecki WT, Futscher BW, Grogan TM, Dalton WS. P-glycoprotein expression and function in circulating blood cells from normal volunteers.. Blood. 1994; 83(9):2451-8. (Biology). View Reference
-
List AF. Role of multidrug resistance and its pharmacological modulation in acute myeloid leukemia.. Leukemia. 1996; 10(6):937-42. (Biology). View Reference
-
Shi T, Wrin J, Reeder J, Liu D, Ring DB. High-affinity monoclonal antibodies against P-glycoprotein. Clin Immunol Immunopathol. 1995; 76(1):44-51. (Immunogen: ELISA, Flow cytometry, Immunoprecipitation). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.