-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- BD OMICS-One™ WTA Next Assay
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?
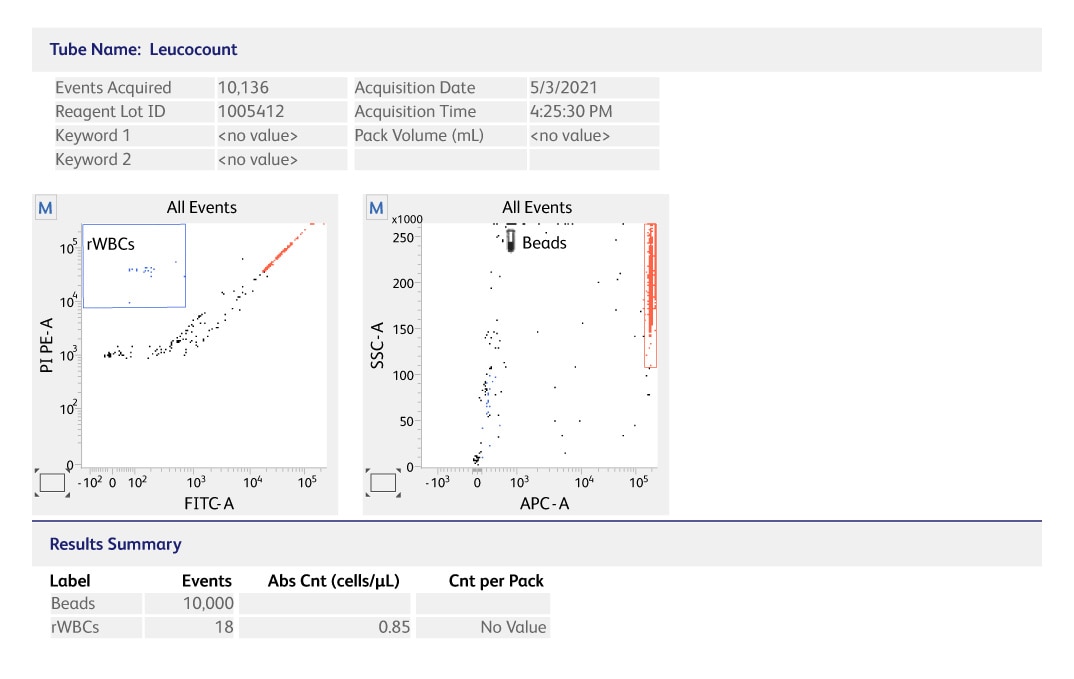
The BD Leucocount™ Kit on the BD FACSLyric™ Flow Cytometer is an CE-IVD assay for the quality control of leucoreduced blood products, which help simplifies the enumeration of residual white blood cells (rWBCs).
Learn more from the BD Leucocount™ Kit brochure.
Features
The trusted performance of the BD Leucocount™ Kit
- For in vitro diagnostic use on the BD FACSLyric™ Flow Cytometer
- Utilizes BD Trucount™ Tube technology for an accurate and reproducible single-platform assay
- Provides equivalent results on the BD FACSLyric™ Flow Cytometer as on the BD FACSVia™ Flow Cytometer
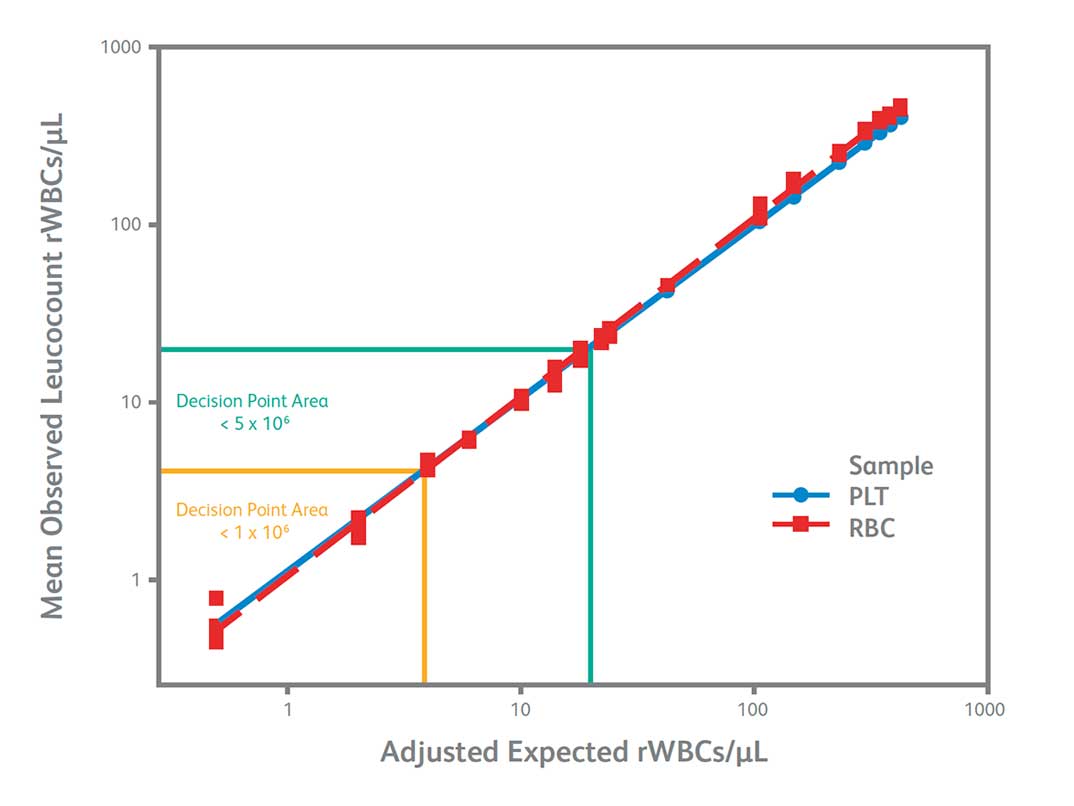
- Offers sensitivity (Limit of Quantitation of 0.7 rWBC cells/µL) that aligns with leukoreduction guidelines
- Delivers linear results over a range from 0.7 – 350 rWBC cells/µL
- Has been validated for age of specimen of up to 48 hours from leucoreduction
The efficient workflow on the BD FACSLyric™ Flow Cytometer
- Enables an efficient and intuitive workflow when used with the BD Leucocount™ Kit and BD Leucocount™ RBC and PLT Controls
- Easily integrated into existing assay menu and workflow on the BD FACSLyric™ Flow Cytometer
- Delivers high-throughput capability with acquisition using the BD FACS™ Universal Loader
- Simplifies analysis with pre-defined templates, lab reports, automated gating and LIS integration
- Features relevant to 21 CFR Part 11 with audit trail and e-signatures
Applications
-
Brochures
-
Instruction for Use
-
Application Guide
![]()
The BD FACSLyric™ Flow Cytometer is a Class 1 Laser Product.
BD FACSLyric™ Flow Cytometer with the BD FACS™ Universal Loader, BD FACSuite™ Clinical and BD FACSuite™ Applications is an in vitro diagnostic medical device bearing a CE mark.
BD Leucocount™ Kit and BD Leucocount™ RBC and PLT Controls are in vitro diagnostic medical devices bearing a CE mark and are CE certified by BSI Group the Netherlands B.V.