IVDR – Big Change is on the Horizon. Are You Ready?
The importance of laboratory diagnostics
April 17, 2024
Many clinical decisions at diagnosis and follow up are dependent on pathology laboratory test results. If inaccurate results are reported, the consequences can have significant impact such as; unnecessary treatment, treatment complications, failure to provide appropriate treatment, diagnosis delays and unnecessary additional diagnostic testing.1 It is therefore critical that diagnostic laboratory testing is accurate, reliable, of the best quality and, of course, timely to ensure that patients receive the correct diagnosis and the most effective, timely treatment. Accurate diagnostics and subsequent treatment decisions are dependent upon standardised testing to produce consistent, timely and quality results.1 Error-prone steps can impact the laboratory workflow and if errors occur, they can have a major impact on diagnostic and treatment pathways.1,2,3
Why IVDR?
Laboratory testing plays a fundamental role in clinical decision making and can have a significant impact on patient safety and health outcomes1 and is now becoming increasingly important.4 It follows that the regulation governing in vitro diagnostic medical devices used by health institutions, like labs*, which are manufacturing, modifying and using in-house devices, should ensure a framework that sets high standards of quality with the aim of providing a high level of health protection for patients. EU-IVDR was not only an update of the IVD Directive 98/79/EC, but an in-depth change of the rules applicable for placing in vitro diagnostic medical devices on the market. IVDR impacts both manufacturers and health institutions and so we are very much in this together.
*Labs within hospitals or laboratories and public health institutes that support the health care system and/ or address patient needs, but which do not treat or care for patients directly
When?
The transition to IVDR started in 2017 and in the end, it will take over 10 years to be fully implemented. We have already passed key milestones, but significant other milestones are on the horizon and these are important to health institutions, like labs, as well.

*Article 5(5) a: transfer of devices
** Article 5(5) b: QMS, Article 5(5) c: EN ISO 15189 compliance or where applicable, national provisions, Article 5(5) e: Information to competent authority, Article 5(5) f: Public declaration, Article 5(5) g: documentation Class D (member states may extend to class A, B or C), Article 5(5) h: Manufacturing in accordance with point g, Article 5(5) i: Review clinical experience
*** Including Article 5(5) d: Justification for use
Impact to manufacturers
Companies have to meet enhanced requirements to support the safety and performance of their devices, which are subject to greater scrutiny by notified bodies and competent authorities. More information can be found here about how BD have prepared and continue to innovate to ensure a robust IVDR compliant portfolio to support haemato-oncology, immunology and transfusion and transplantation laboratories (link to FF LP).
What about Laboratory Developed Tests (LDTs)?
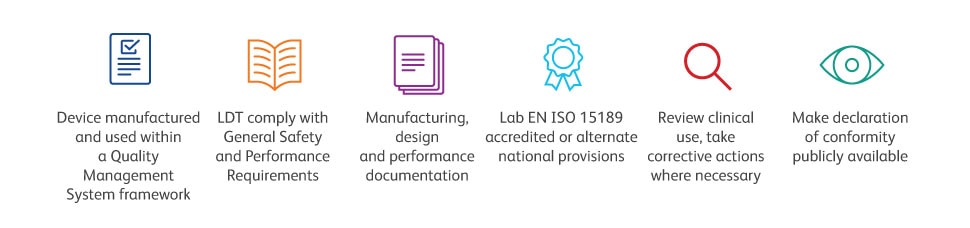
When a suitable IVDR compliant device is not available, health institutions can manufacture and use in-house in vitro diagnostics (IH-IVDs). However, considerable investment in resources is required, to ensure that firstly, these IH-IVDs are compliant and safe and secondly, that testing can continue, minimising impact to patients. This is particularly applicable to testing for rare diseases where there is no CE-IVD equivalent4. In order to benefit from the exemption of having to comply with all applicable requirements of the IVDR (EU) 2017/746, the health institution must meet all Article 5(5) conditions so the IH-IVD only needs to meet the relevant safety and performance requirements as outlined in Annex I.4, 5 Compliance with Annex I has been applicable since May 2022. Now labs should be gearing up to ensure that they also meet the other conditions within Article 5(5), with the exception of part d, by 26th May 2024. Under Article 5(5), there are 9 conditions listed that must be met and some of the key ones are shown here.
Key requirements addressing LDTs within health institutions as per Article 5(5) of the EU-IVDR
Note: The above lists just a few of the conditions as outlined in Article 5(5)
Please note that ALL conditions as outlined under Article 5(5) need to be met for the exemption to apply
In fact, many labs are already meeting some of the Article 5(5) conditions today for example, following a QMS, accredited according to ISO 15189. Reassuringly, labs that are already ISO15189 compliant have solid foundations in place to build upon to support transition to IVDR compliance4. In addition, labs should prepare documentation on manufacturing, design and performance (a requirement for Class D devices that may be extended to Class A, B and C devices by Member States), to justify the use of an IH-IVD and demonstrate to have risk management process in place.5
For the first time, labs will need to justify the use of IH-IVDs and they can only be used, if there is no equivalent CE-IVD device on the market or if they can demonstrate that a target patient-group’s specific needs cannot be met, or cannot be met at the appropriate level of performance by an equivalent CE-IVD device. The deadline for demonstrating this has been postponed to 26th May 2028 (IVDR Amending Regulation (EU) 2022/112).
Impact to labs
Due to the additional workload in achieving compliance, the number of IH-IVDs performed by labs is likely to decrease.4 A recent report published by the Biomedical Alliance in Europe, who created an IVDR task force and represent more than 400,000 healthcare professionals, highlights the need for urgent action to prevent shortage of diagnostic tests (both CE-marked and In-House IVDs).6 They summarise widespread issues with the implementation of IVDR and among others are calling out the specific impact of Article 5(5) which discourages the development of IH-IVDs and recommend that the European Commission, amongst other key recommendations, grant an urgent extension to the transition periods.6 Despite this, labs should continue to prepare for the May 2024 and May 2028 deadlines. The full report is available.
Meanwhile, a new ISO standard, ISO/DIS 5649 is currently under development and specifies the design, development, implementation and use of laboratory developed tests.7
BD can help
BD are committed to delivering IVDR compliant solutions that are safe and effective to serve you and your patients. We received our first IVDR product certification back in December 2020 and the majority of BD’s previous IVD Directive 98/79/EC compliant clinical flow cytometry devices are now IVDR compliant. We are continuing our efforts to achieve this for current and new innovative products in our clinical portfolio.
One integrated walkaway sample-to-answer solution
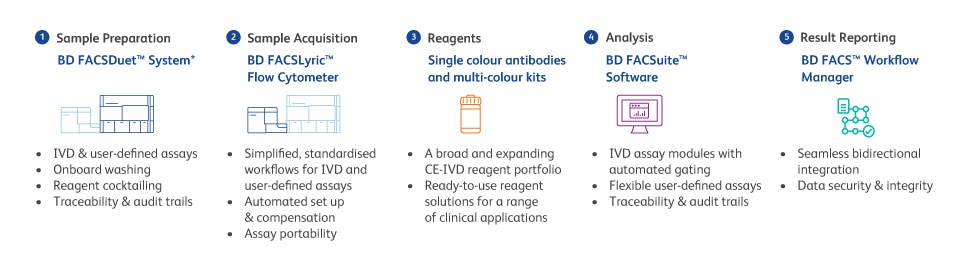
* BD FACSDuet™ Sample Preparation System and BD FACSDuet™ Premium Sample Preparation System
Concluding remarks
A IVDR aims to set high standards of safety and quality for CE-IVD and IH-IVDs. Considerable investment (e.g. dedicated resources) and an eye on the timelines are required to ensure IVDR compliance and service continuity. BD have an extensive compliant portfolio to support flow cytometry within haemato-oncology, immunology and transfusion and transplantation laboratories with CE-IVD solutions and an expanding portfolio of single colour CE-IVD reagents. We offer a complete integrated clinical flow cytometry solution that simplifies and automates processes, with traceability and electronic audit trails, helping to drive compliance, automation, standardisation and quality. The BD clinical solution can help to keep moving forward on your journey to IVDR.
Download the IVDR Brochure>