Overview
Flow cytometry has been widely used for decades as a highly sensitive tool for immunophenotypic analysis and is playing an increasing role in biomarker detection in clinical trials, e.g. in the expanding clinical trials landscape in oncohaematology, including CAR-T clinical trials.1 Next Generation Flow™ (NGF) for MRD (Minimal Residual Disease) represents one of the latest advancements in this powerful technology, enhancing precision, efficiency, and standardisation to deliver peerless reproducible results. Clinical research investigating immune mechanisms frequently uses immune assessment to understand the processes of immunotherapeutic approaches.
BD as a Partner
BD offers integrated solutions from sample preparation to data analysis to provide accurate, consistent results in your clinical development program. The need for standardised, certified techniques that can demonstrate compliance with regulations is essential to achieve consistency and efficiency, reduce error and inter-operator variability, and enable reproducibility over multiple centres.
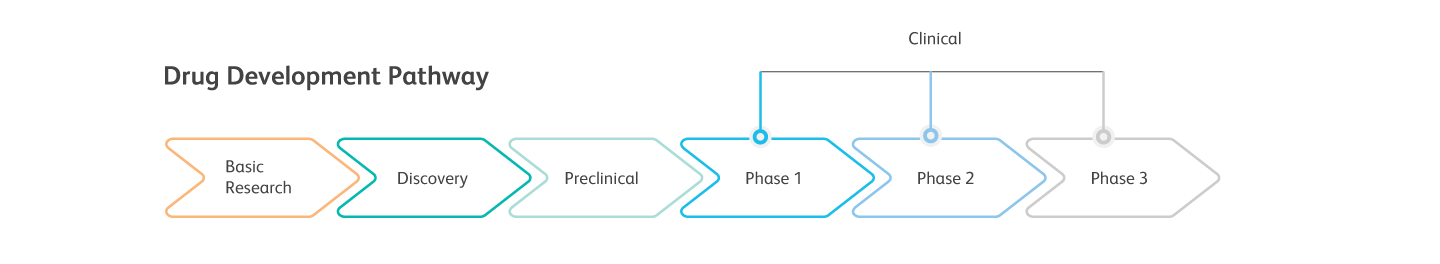
Discover the benefits of Next Generation Flow™ for MRD measurements in BCP-ALL* and Multiple Myeloma
Next Generation Flow™ (NGF) makes it possible to rapidly acquire several millions of cells and therefore reach the sensitivity of molecular methods. Standardization at every step of the process, coupled with innovative software tools, yields accurate, reproducible and objective results.2
The rapid and precise performance of NGF can therefore offer advantages in a clinical trial setting for MRD evaluation:
- High sensitivity comparable to PCR techniques: NGF reaches sensitivities up to 10-6 2
- Wide applicability: No primers or diagnostic samples are required3
- Fast turnaround time: Results are obtained within hours of the sample being received 3
- Information on population context: For example, heterogeneity of the whole population can be identified2
*BCP-ALL: B-cell precursor acute lymphoblastic leukaemia
Maximise Result Reproducibility, Process Traceability, and Workflow Efficiency with Improved Standardisation and Automation
Standardisation of all process steps is of added value in clinical trials to reduce inter-operator variability during processing and analysis of samples and to reduce error prone steps, thereby enabling reproducibility of results in an efficient manner across multi-centre studies.
Automation allows you to reduce hands-on time, decrease operator variability, and reduce time for documentation due to automated processes enabling sample and reagent traceability.
Clinical trials are run in a regulated environment where different compliance related topics can be relevant, like Fit-for-purpose validation, support of 21 CFR part 11 features and IVDR depending on the nature of the trial.

Why choose BD Next Generation Flow™ solutions for BCP-ALL and Multiple Myeloma MRD evaluation?
NGF as a technique for BCP-ALL and Multiple Myeloma MRD evaluation offers multiple benefits in a clinical trial setting:
- CE-IVD labeled reagent kits
- Methodology is scientifically validated by EuroFlow™
- Higher sensitivity than conventional 8-colour Flow MRD4,5
- The methodology (panel + standard operating procedure + Bulklysis™ protocol) results are significantly high concordant to PCR-based techniques (BCP-ALL MRD) and highly concordant and complementary to NGS techniques (multiple myeloma MRD)
- Provides information faster (within the same day) than PCR-based and NGS techniques4,5
- Compliant with FDA guidance for the use of MRD in the development of drug and biological products for treatment6
- Complete, ready-to-use kit increases efficiency and reduces error
BCP-ALL MRD
Acute lymphoblastic leukaemia (ALL) is the first neoplasm in which the evaluation of the initial response to treatment through the assessment of MRD has proven to be a fundamental tool to manage therapeutic decisions.7 High quality MRD assessment data demonstrating the efficacy of new and emerging treatments (such as targeted therapy e.g., bi-specific AB, CAR-T therapy) is needed if these treatments are to be made available and more patients are to benefit.
- Applicable in >98% of samples as there is no requirement for specific primers and probes7
- High sensitivity close to 10-5 (Bulklysis™ protocol and acquisition of 4 million events per tube) 7
- Can be used to detect pathological B-cell precursors in patients who have been treated with anti-CD19 therapies8
- The antibody panel maximizes the differences between pathological B-cell precursors and their normal counterparts7

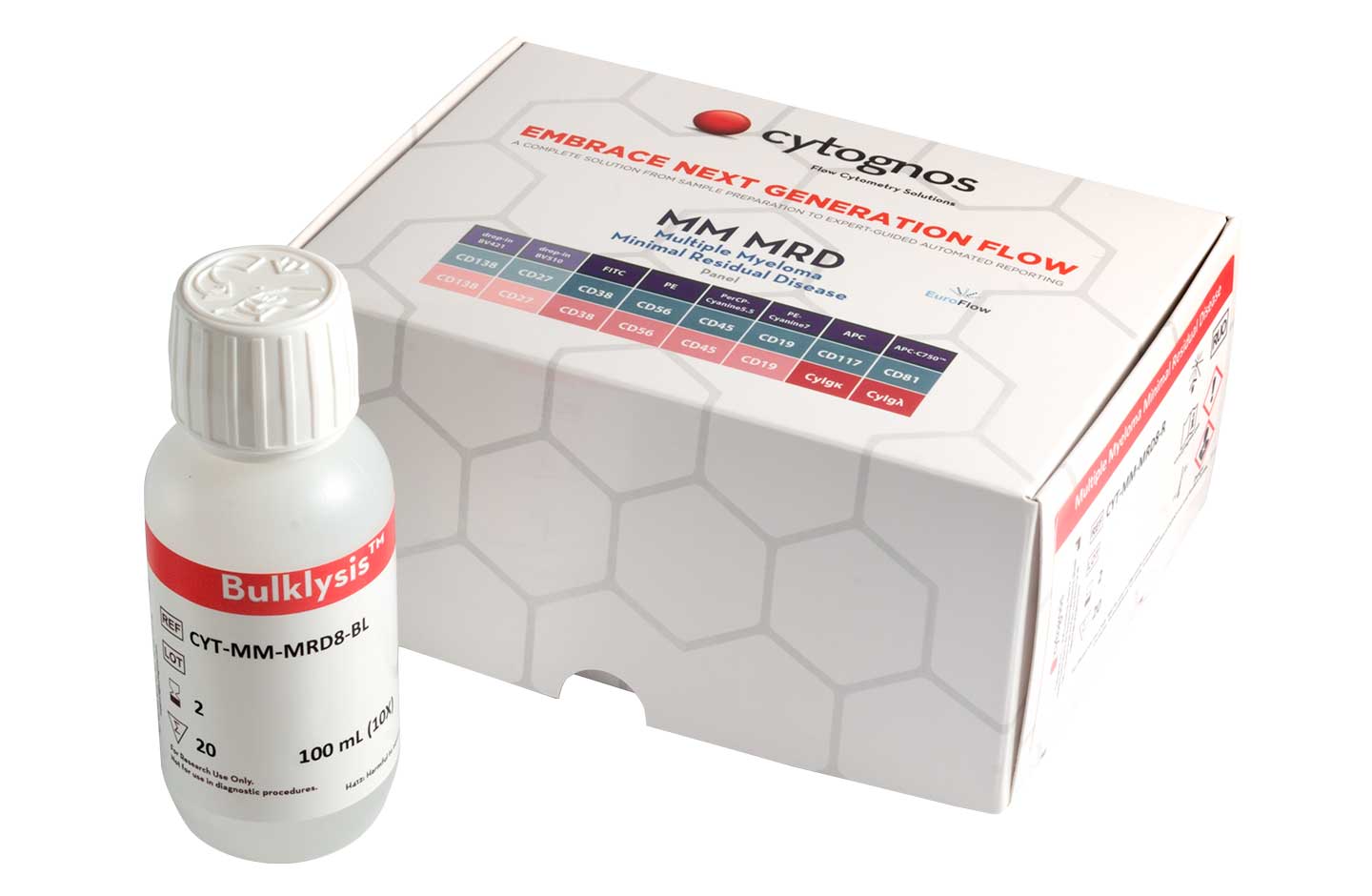
MM MRD
New drugs and techniques have assisted the improvement in progression-free survival over the past ten years for those suffering from multiple myeloma (MM); however, a number of patients who respond well to treatment still relapse due to MRD.9 The NGF methodology developed by EuroFlow™ for the detection of MRD in MM is endorsed by the International Myeloma Working Group, which included it as a technique of choice in their last review of the response assessment criteria.10
- High sensitivity close to 10-6 (Bulklysis™ protocol and acquisition of 5 million events per tube)5
- Maximises the differences between normal plasma cells and pathological ones5
- Detects pathological cells in patients treated with anti-CD38 therapies due to the addition of CD38 multi-epitope5
Characterisation of Circulating Plasma Cells for exploratory clinical trial endpoints
CPC antibody panel combination can be used to study plasma cells in peripheral blood in flow cytometry.
The CPC kit is designed following the EuroFlow™ 8-colour antibody panel.11
The CYT-CPC panel is for Research Use Only. Not for use in diagnostic or therapeutic procedures.
BD Infinicyt™ software is designed for multiparametric analysis of complex flow cytometry data with patented tools and adaptable to common laboratory workflows:
- Complete standardised and validated CE-IVD solution, important in the clinical trial context
- Detection and diagnosis of onco-haematological diseases and primary immunodeficiencies, and assessment of the immune system
- Advanced multiparametric manual analysis for complex analyses
- Unique software including reference databases: EuroFlow™ databases enable automated analysis with validated reference databases allows standardisation of the process, providing reproducible and objective results
- The 21 Code of Federal Regulations (CFR) part 11 BD Infinicyt™ features ensure traceability and security of data thereby meeting important security requirements
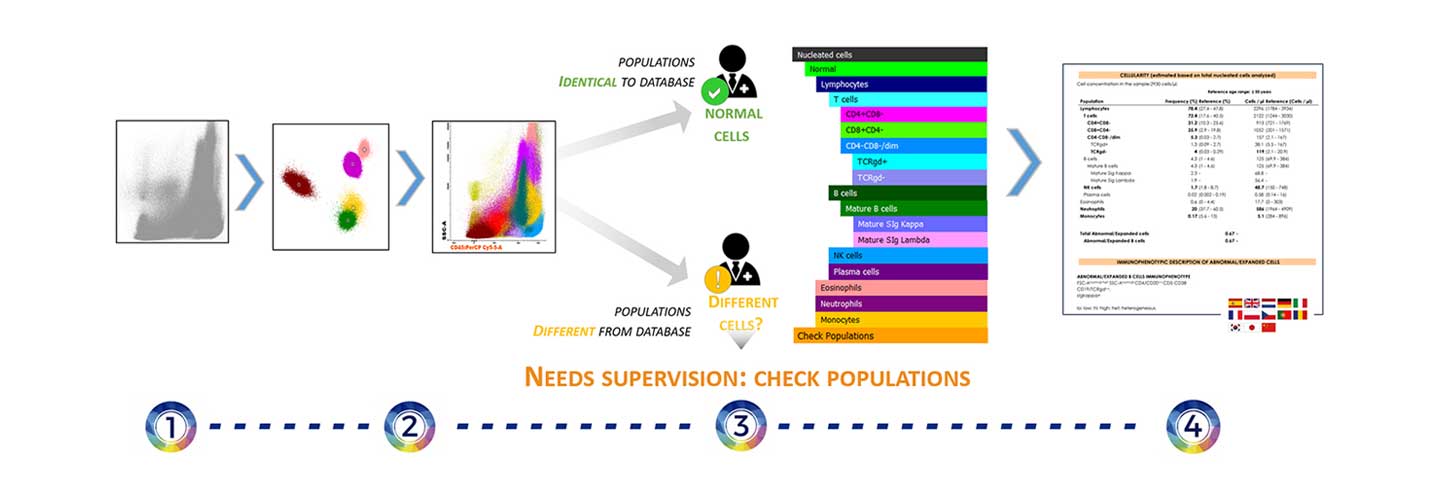
Automated analysis of a sample process. Events are first grouped into clusters to be compared with the reference populations classified in the database. After comparison, populations that have similar characteristics to the reference are automatically classified. The user then reviews the events to decide if there are pathological cells in the sample. All information will be reflected in a results report.
Achieve reproducibility of results across instruments and sites through standardisation of instrument setup with the BD FACSLyric™ Flow Cytometer
A large global install base of BD’s IVDR-compliant BD FACSLyric™ Flow Cytometer allows easy collaboration across industry, clinical trial sites, and academic labs, supporting FDA 21 Code of Federal Regulations (CFR) Part 11 features and enabling electronic portability of user-defined assays.
The BD FACSLyric™ Flow Cytometer has an install base of more than 3,000 units globally
Reproducibility of results across instruments and sites through standardisation of instrument setup with the BD FACSLyric™ Flow Cytometer
Features that facilitate your setup of clinical trials:
- Built-in daily quality control steps automate several previously manual steps, increasing reproducibility and standardisation.
- BD FACS™ Universal loader offers automation and flexibility in sample input, from tubes to 96 and 384 multiwell plates (21 different options).
- User-defined electronic assay portability of the BD FACSuite™ Application simplifies and standardises instrument setup, easing the transfer of assays to multiple trial sites and reducing validation effort.
- Audit trail and electronic signature support 21 CFR Part 11 features, which is particularly useful in good manufacturing practice (GMP) environments.
- Installation qualification, operational qualification, and guaranteed technical service provided by BD to minimise instrument downtime.
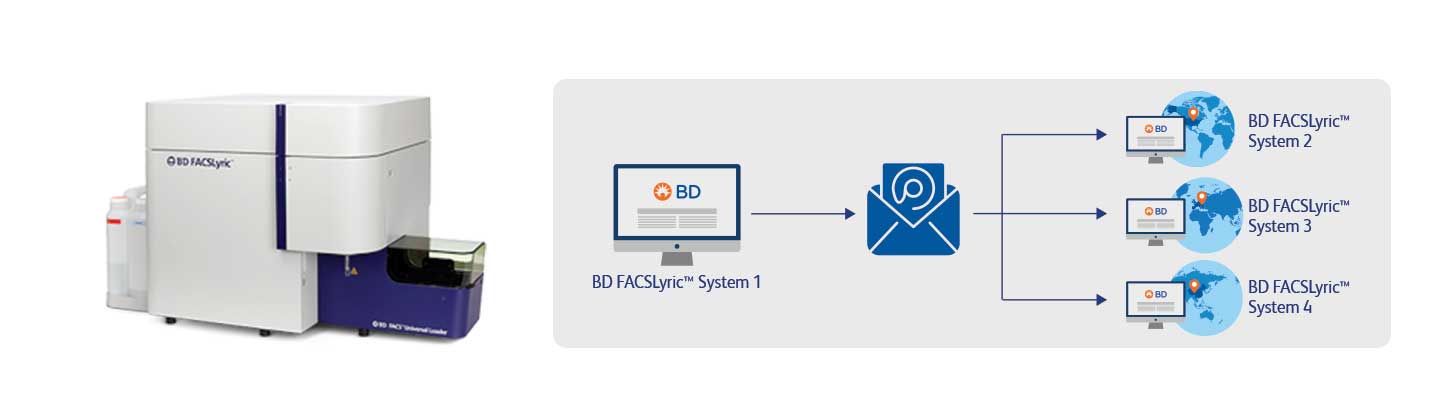
Increase workflow efficiency through automation with BD FACSDuet™ Sample Preparation System
The BD FACSDuet™ Sample Preparation System is a comprehensive system that provides a complete walkaway workflow solution offering automatic sample transfer through physical integration with the BD FACSLyric™ Flow Cytometer. This helps drive standardisation, consistency, and data reproducibility for both IVD and user-defined assays. The BD FACSDuet™ Premium System further enhances efficiency with optional on-board washing and centrifugation, accommodating a variety of assays, reducing hands-on time, and helping maximise resources for both Lyse-No-Wash and Lyse-Wash-assays. The system offers flexibility for different sizes and tubes of specimens. For Lyse-No-Wash assays 96-well plates are enabled.
Reagent cocktailing functionality allows on-board automated antibody cocktail preparation, eliminating the risk of errors in manual pipetting and driving automated recording of all used antibodies for easier tracking and auditing. With the use of vial adaptors, the system enables compatibility with a wide variety of reagent vials from a range of manufacturers. The system delivers complete traceability, with easy-to-pull reports of specimens, worklists, and reagents, facilitating inventory and data management. Features related to 21 CFR Part 11 are supported with an audit trail.
Effectively assess immune response with integrated BD solutions
- Clinical research investigating immune mechanisms frequently uses immune assessment to understand the processes of the immune system. BD Multitest™ Reagents with optional BD Trucount™ Tubes offer a choice of different IVDR compliant combinations for identification and enumeration (percentage and absolute counts) of total T cell, B cell and NK cell lymphocyte subsets. Complete the integrated solution with the BD FACSDuet™ Sample Preparation System, allowing a complete automated workflow from sample to answer to help reduce hands-on time and error-prone steps.
Deeper lymphocyte subsets can be studied with the CE-IVD Primary Immunodeficiency Orientation Tube (PIDOT), based on the scientific validation work of the EuroFlow™ Consortium using the Next Generation Flow™ approach. This enables greater process standardisation and higher sensitivity as compared to classical flow cytometry.
- Our IVDR single color reagent portfolio adds flexibility where needed for user-defined tests. BD FACSuite™ Application for user-defined assays with its unique assay portability enables easy transfer of assays to multiple sites facilitating efficient collaboration globally.
Ensure Robust Regulatory Compliance and Assay Validation for Clinical Trials
Flow Cytometry Validation Strategy and Fit-for-Purpose Guidelines
The Clinical and Laboratory Standards Institute (CLSI) recently published guideline H62, which focuses on the unique ‘fit-for-purpose’ requirements for the analytic validation of cell-based assays performed by flow cytometry in a clinical trial setting.12 The recommended validation strategy depends on the intended use of the flow cytometry data and its associated level of clinical risk. In a clinical trial, where the intended use of the data is for end points not related to patient care or treatment, the assay would be considered low clinical risk. Clinical trial biomarker assays used to determine enrolment into a clinical trial and therapeutic intervention may be considered high clinical risk.12
In Vitro Diagnostic Medical Device Regulations impact manufacturers and users
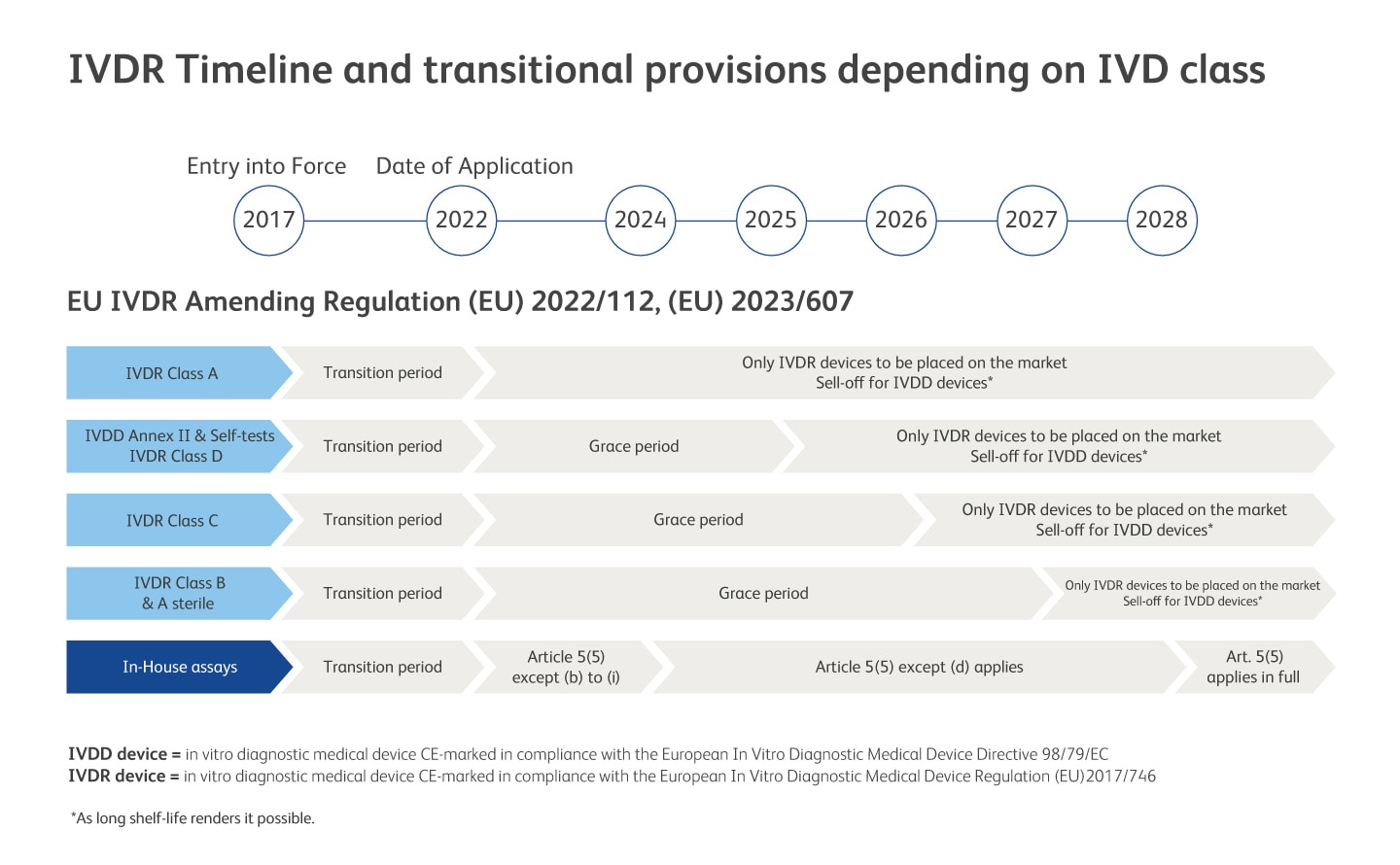
The European In Vitro Diagnostic Medical Device Regulation (IVDR) (EU) 2017/746 came into effect on 26 May 2017 and replaces the European In Vitro Diagnostic Medical Device Directive (IVDD) 98/79/EC.13EU IVDR Amending Regulations (EU) 2022/112 and (EU) 2023/607 expand the scope of the grace period to certain devices and the abolishment of the sell-off provisions of IVDR. We received our first IVDR product certification back in December 2020 and the majority of BD’s previous IVDD compliant devices are now IVDR compliant. We are continuing our efforts to achieve this for current and new products in our clinical portfolio.
The Medical Device Coordination Group (MDCG) 2022-10 document outlines that an assay in the context of a clinical trial might fulfil the definition of an IVD medical device according to IVD Regulation 2017/746 Article 2.14
This states that, when a medical decision is involved, e.g., for inclusion and exclusion of subjects, treatment allocation, or monitoring the safety of the treatment during the trial, it is the responsibility of the clinical trial sponsor to determine the regulatory status of the assay based on the planned use in the trial.13
Companion Diagnostic (CDx) is in scope of IVDR, CDx devices will have to meet all applicable requirements as laid down in the IVDR. Built on a foundation of excellence and immunological expertise, BD Biosciences is committed to unlocking the potential of flow cytometry–based CDx solutions.
BD is Your Dependable Partner for CDx
BD is an excellent partner for CDx development based on acknowledged diagnostic expertise:
- Deep commercial experience with flow cytometry
- Dedicated CDx team
- Global install base
- Regulatory compliance (IVD/IVDR)
- End-to-end solutions
- Minimal Residual Disease Evaluation for BCP-ALL and MM
Our Next Generation Flow™ (NGF*) MRD detection solution for BCP-ALL and multiple myeloma, respectively, is an advanced method of multicolour flow cytometry that rapidly alerts clinicians if the disease is still present or if there is a sign of recurrence. The solution allows the simultaneous analysis of several phenotypic markers on up to 107 cells, showing detection limits comparable to the most sensitive molecular techniques.
- BD Infinicyt ™
The BD Infinicyt™ Software was designed for multiparametric analysis of complex data, providing CE-IVD manual and automated data analysis and reporting when working with the intended uses of the appropriate BD CE-IVD panels for a comprehensive evaluation of pathological and rare cells to achieve the appropriate sensitivity.
- BD Multitest™ Reagents
BD Multitest™ Reagents with optional BD Trucount™ Tubes offer a choice of different IVDR compliant combinations for identification and enumeration of total T cell, B cell and NK lymphocyte subsets. Deeper lymphocyte subsets can be studied with the CE-IVD Primary Immunodeficiency Orientation Tube (PIDOT).
- BD FACSLyric™ Flow Cytometer
The BD FACSLyric™ Flow Cytometer combines simplicity, speed and automation to ease workload and improve productivity. This next-generation system enables flow cytometry workflow standardisation and collaboration through automation and unique assay portability capabilities.
- BD FACSDuet™ Sample Preparation System
The BD FACSDuet™ Sample Preparation System and BD FACSDuet™ Premium Sample Preparation System paired with the BD FACSLyric™ Flow Cytometry System delivers process standardisation by minimising error-prone steps while increasing workflow consistency and laboratory efficiency.
- Custom Dried Multicolour Panels (RUO)
Custom dried multicolour panels (RUO) can help to reduce inter-experiment variability and come with long shelf lives making them ideal choices for multisite and longitudinal studies.
- CE-IVD labeled reagent kits
- Methodology is scientifically validated by EuroFlow™
- Higher sensitivity than conventional 8-colour Flow MRD4,5
- The methodology (panel + standard operating procedure + Bulklysis™ protocol) results are significantly high concordant to PCR-based techniques (BCP-ALL MRD) and highly concordant and complementary to NGS techniques (multiple myeloma MRD)
- Provides information faster (within the same day) than PCR-based and NGS techniques4,5
- Compliant with FDA guidance for the use of MRD in the development of drug and biological products for treatment6
- Complete, ready-to-use kit increases efficiency and reduces error
- Applicable in >98% of samples as there is no requirement for specific primers and probes7
- High sensitivity close to 10-5 (Bulklysis™ protocol and acquisition of 4 million events per tube) 7
- Can be used to detect pathological B-cell precursors in patients who have been treated with anti-CD19 therapies8
- The antibody panel maximizes the differences between pathological B-cell precursors and their normal counterparts7
- High sensitivity close to 10-6 (Bulklysis™ protocol and acquisition of 5 million events per tube)5
- Maximises the differences between normal plasma cells and pathological ones5
- Detects pathological cells in patients treated with anti-CD38 therapies due to the addition of CD38 multi-epitope5
- Complete standardised and validated CE-IVD solution, important in the clinical trial context
- Detection and diagnosis of onco-haematological diseases and primary immunodeficiencies, and assessment of the immune system
- Advanced multiparametric manual analysis for complex analyses
- Unique software including reference databases: EuroFlow™ databases enable automated analysis with validated reference databases allows standardisation of the process, providing reproducible and objective results
- The 21 Code of Federal Regulations (CFR) part 11 BD Infinicyt™ features ensure traceability and security of data thereby meeting important security requirements
Why choose BD Next Generation Flow™ solutions for BCP-ALL and Multiple Myeloma MRD evaluation?
NGF as a technique for BCP-ALL and Multiple Myeloma MRD evaluation offers multiple benefits in a clinical trial setting:
BCP-ALL MRD
Acute lymphoblastic leukaemia (ALL) is the first neoplasm in which the evaluation of the initial response to treatment through the assessment of MRD has proven to be a fundamental tool to manage therapeutic decisions.7 High quality MRD assessment data demonstrating the efficacy of new and emerging treatments (such as targeted therapy e.g., bi-specific AB, CAR-T therapy) is needed if these treatments are to be made available and more patients are to benefit.

MM MRD
New drugs and techniques have assisted the improvement in progression-free survival over the past ten years for those suffering from multiple myeloma (MM); however, a number of patients who respond well to treatment still relapse due to MRD.9 The NGF methodology developed by EuroFlow™ for the detection of MRD in MM is endorsed by the International Myeloma Working Group, which included it as a technique of choice in their last review of the response assessment criteria.10
Characterisation of Circulating Plasma Cells for exploratory clinical trial endpoints
CPC antibody panel combination can be used to study plasma cells in peripheral blood in flow cytometry.
The CPC kit is designed following the EuroFlow™ 8-colour antibody panel.11
The CYT-CPC panel is for Research Use Only. Not for use in diagnostic or therapeutic procedures.

BD Infinicyt™ software is designed for multiparametric analysis of complex flow cytometry data with patented tools and adaptable to common laboratory workflows:

Automated analysis of a sample process. Events are first grouped into clusters to be compared with the reference populations classified in the database. After comparison, populations that have similar characteristics to the reference are automatically classified. The user then reviews the events to decide if there are pathological cells in the sample. All information will be reflected in a results report.
- Built-in daily quality control steps automate several previously manual steps, increasing reproducibility and standardisation.
- BD FACS™ Universal loader offers automation and flexibility in sample input, from tubes to 96 and 384 multiwell plates (21 different options).
- User-defined electronic assay portability of the BD FACSuite™ Application simplifies and standardises instrument setup, easing the transfer of assays to multiple trial sites and reducing validation effort.
- Audit trail and electronic signature support 21 CFR Part 11 features, which is particularly useful in good manufacturing practice (GMP) environments.
- Installation qualification, operational qualification, and guaranteed technical service provided by BD to minimise instrument downtime.
- Clinical research investigating immune mechanisms frequently uses immune assessment to understand the processes of the immune system. BD Multitest™ Reagents with optional BD Trucount™ Tubes offer a choice of different IVDR compliant combinations for identification and enumeration (percentage and absolute counts) of total T cell, B cell and NK cell lymphocyte subsets. Complete the integrated solution with the BD FACSDuet™ Sample Preparation System, allowing a complete automated workflow from sample to answer to help reduce hands-on time and error-prone steps.
Deeper lymphocyte subsets can be studied with the CE-IVD Primary Immunodeficiency Orientation Tube (PIDOT), based on the scientific validation work of the EuroFlow™ Consortium using the Next Generation Flow™ approach. This enables greater process standardisation and higher sensitivity as compared to classical flow cytometry.
- Our IVDR single color reagent portfolio adds flexibility where needed for user-defined tests. BD FACSuite™ Application for user-defined assays with its unique assay portability enables easy transfer of assays to multiple sites facilitating efficient collaboration globally.
Achieve reproducibility of results across instruments and sites through standardisation of instrument setup with the BD FACSLyric™ Flow Cytometer
A large global install base of BD’s IVDR-compliant BD FACSLyric™ Flow Cytometer allows easy collaboration across industry, clinical trial sites, and academic labs, supporting FDA 21 Code of Federal Regulations (CFR) Part 11 features and enabling electronic portability of user-defined assays.

The BD FACSLyric™ Flow Cytometer has an install base of more than 3,000 units globally
Reproducibility of results across instruments and sites through standardisation of instrument setup with the BD FACSLyric™ Flow Cytometer
Features that facilitate your setup of clinical trials:

Increase workflow efficiency through automation with BD FACSDuet™ Sample Preparation System
The BD FACSDuet™ Sample Preparation System is a comprehensive system that provides a complete walkaway workflow solution offering automatic sample transfer through physical integration with the BD FACSLyric™ Flow Cytometer. This helps drive standardisation, consistency, and data reproducibility for both IVD and user-defined assays. The BD FACSDuet™ Premium System further enhances efficiency with optional on-board washing and centrifugation, accommodating a variety of assays, reducing hands-on time, and helping maximise resources for both Lyse-No-Wash and Lyse-Wash-assays. The system offers flexibility for different sizes and tubes of specimens. For Lyse-No-Wash assays 96-well plates are enabled.
Reagent cocktailing functionality allows on-board automated antibody cocktail preparation, eliminating the risk of errors in manual pipetting and driving automated recording of all used antibodies for easier tracking and auditing. With the use of vial adaptors, the system enables compatibility with a wide variety of reagent vials from a range of manufacturers. The system delivers complete traceability, with easy-to-pull reports of specimens, worklists, and reagents, facilitating inventory and data management. Features related to 21 CFR Part 11 are supported with an audit trail.

Effectively assess immune response with integrated BD solutions
- Deep commercial experience with flow cytometry
- Dedicated CDx team
- Global install base
- Regulatory compliance (IVD/IVDR)
- End-to-end solutions
Ensure Robust Regulatory Compliance and Assay Validation for Clinical Trials
Flow Cytometry Validation Strategy and Fit-for-Purpose Guidelines
The Clinical and Laboratory Standards Institute (CLSI) recently published guideline H62, which focuses on the unique ‘fit-for-purpose’ requirements for the analytic validation of cell-based assays performed by flow cytometry in a clinical trial setting.12 The recommended validation strategy depends on the intended use of the flow cytometry data and its associated level of clinical risk. In a clinical trial, where the intended use of the data is for end points not related to patient care or treatment, the assay would be considered low clinical risk. Clinical trial biomarker assays used to determine enrolment into a clinical trial and therapeutic intervention may be considered high clinical risk.12
In Vitro Diagnostic Medical Device Regulations impact manufacturers and users
The European In Vitro Diagnostic Medical Device Regulation (IVDR) (EU) 2017/746 came into effect on 26 May 2017 and replaces the European In Vitro Diagnostic Medical Device Directive (IVDD) 98/79/EC.13EU IVDR Amending Regulations (EU) 2022/112 and (EU) 2023/607 expand the scope of the grace period to certain devices and the abolishment of the sell-off provisions of IVDR. We received our first IVDR product certification back in December 2020 and the majority of BD’s previous IVDD compliant devices are now IVDR compliant. We are continuing our efforts to achieve this for current and new products in our clinical portfolio.
The Medical Device Coordination Group (MDCG) 2022-10 document outlines that an assay in the context of a clinical trial might fulfil the definition of an IVD medical device according to IVD Regulation 2017/746 Article 2.14
This states that, when a medical decision is involved, e.g., for inclusion and exclusion of subjects, treatment allocation, or monitoring the safety of the treatment during the trial, it is the responsibility of the clinical trial sponsor to determine the regulatory status of the assay based on the planned use in the trial.13

Companion Diagnostic (CDx) is in scope of IVDR, CDx devices will have to meet all applicable requirements as laid down in the IVDR. Built on a foundation of excellence and immunological expertise, BD Biosciences is committed to unlocking the potential of flow cytometry–based CDx solutions.
BD is Your Dependable Partner for CDx
BD is an excellent partner for CDx development based on acknowledged diagnostic expertise:
- Minimal Residual Disease Evaluation for BCP-ALL and MM
Our Next Generation Flow™ (NGF*) MRD detection solution for BCP-ALL and multiple myeloma, respectively, is an advanced method of multicolour flow cytometry that rapidly alerts clinicians if the disease is still present or if there is a sign of recurrence. The solution allows the simultaneous analysis of several phenotypic markers on up to 107 cells, showing detection limits comparable to the most sensitive molecular techniques.
- BD Infinicyt ™
The BD Infinicyt™ Software was designed for multiparametric analysis of complex data, providing CE-IVD manual and automated data analysis and reporting when working with the intended uses of the appropriate BD CE-IVD panels for a comprehensive evaluation of pathological and rare cells to achieve the appropriate sensitivity.
- BD Multitest™ Reagents
BD Multitest™ Reagents with optional BD Trucount™ Tubes offer a choice of different IVDR compliant combinations for identification and enumeration of total T cell, B cell and NK lymphocyte subsets. Deeper lymphocyte subsets can be studied with the CE-IVD Primary Immunodeficiency Orientation Tube (PIDOT).
- BD FACSLyric™ Flow Cytometer
The BD FACSLyric™ Flow Cytometer combines simplicity, speed and automation to ease workload and improve productivity. This next-generation system enables flow cytometry workflow standardisation and collaboration through automation and unique assay portability capabilities.
- BD FACSDuet™ Sample Preparation System
The BD FACSDuet™ Sample Preparation System and BD FACSDuet™ Premium Sample Preparation System paired with the BD FACSLyric™ Flow Cytometry System delivers process standardisation by minimising error-prone steps while increasing workflow consistency and laboratory efficiency.
- Custom Dried Multicolour Panels (RUO)
Custom dried multicolour panels (RUO) can help to reduce inter-experiment variability and come with long shelf lives making them ideal choices for multisite and longitudinal studies.
BD Flow Cytometers, BD FACSDuet™ Sample Preparation System and BD FACSDuet™ Premium Sample Preparation System are Class 1 Laser Products.
![]() BD FACSDuet™ Sample Preparation System, BD FACSDuet™ Premium Sample Preparation System, BD FACSLyric™ Flow Cytometer with the BD FACSuite™ Clinical and BD FACSuite™ Application, Bulklysis™, BCP-ALL MRD Panel, MM MRD Panel, PIDOT Panel and BD Infinicyt™ analysis software are in vitro diagnostic medical devices bearing a CE mark.
BD FACSDuet™ Sample Preparation System, BD FACSDuet™ Premium Sample Preparation System, BD FACSLyric™ Flow Cytometer with the BD FACSuite™ Clinical and BD FACSuite™ Application, Bulklysis™, BCP-ALL MRD Panel, MM MRD Panel, PIDOT Panel and BD Infinicyt™ analysis software are in vitro diagnostic medical devices bearing a CE mark.
![]() BD Multitest™ Reagents (excluding 333184, 333185) and BD Trucount™ Tubes are in vitro diagnostic medical devices bearing a CE mark and are CE certified by BSI Group The Netherlands B.V. (Notified Body Number = 2797).
BD Multitest™ Reagents (excluding 333184, 333185) and BD Trucount™ Tubes are in vitro diagnostic medical devices bearing a CE mark and are CE certified by BSI Group The Netherlands B.V. (Notified Body Number = 2797).
CYT-CPC and Custom dried multicolour panels are for Research Use Only. Not for use in diagnostic or therapeutic procedures
The EuroFlow trademark is the property of the EuroFlow Consortium and cannot be reproduced or published without prior written permission from the EuroFlow coordinator (www.euroflow.org).
BD, the BD Logo, FACSDuet, FACSLyric, FACSuite, Infinicyt and Next Generation Flow are trademarks of Becton, Dickinson and Company or its affiliates.
Bulklysis is a trademark of Cytognos. Cytognos S.L. is a 100% subsidiary of BD.
© 2024 BD. All rights reserved. BD-110178 (v1.0) 0124
