Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
For optimal and reproducible results, BD Horizon Brilliant Stain Buffer should be used anytime two or more BD Horizon Brilliant dyes (including BD OptiBuild Brilliant reagents) are used in the same experiment. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. The BD Horizon Brilliant Stain Buffer was designed to minimize these interactions. More information can be found in the Technical Data Sheet of the BD Horizon Brilliant Stain Buffer (Cat. No. 563794).
Product Notices
- This antibody was developed for use in flow cytometry.
- The production process underwent stringent testing and validation to assure that it generates a high-quality conjugate with consistent performance and specific binding activity. However, verification testing has not been performed on all conjugate lots.
- Researchers should determine the optimal concentration of this reagent for their individual applications.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- BD Horizon Brilliant Stain Buffer is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,575,303; 8,354,239.
- BD Horizon Brilliant Ultraviolet 661 is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,227,187; 8,575,303; 8,354,239.
Companion Products
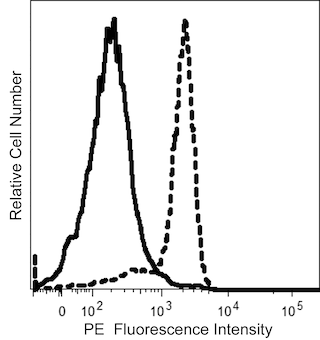




The 134591 monoclonal antibody specifically recognizes CD159 antigen-like family member C (CD159c) which is also known as NKG2-C type II integral membrane protein or NKG2-C-activating NK receptor (NKG2C), or NK cell receptor C. CD159c (NKG2C) is a type II transmembrane glycoprotein that is encoded by KLRC2 (killer cell lectin like receptor C2) which belongs to the killer cell lectin-like receptor (KLR) family. CD159c (NKG2C) contains a C-type lectin domain in its extracellular region and is expressed by NK cells and some T cells. CD159c (NKG2C) can form a disulfide-bonded heterodimer with CD94 that non-covalently associates with DAP12 homodimers to form a functional signaling receptor complex. CD159c (NKG2C) binds to MHC class I HLA-E molecules on target cells to regulate NK cell activation.
The antibody was conjugated to BD Horizon™ BUV661 which is part of the BD Horizon Brilliant™ Ultraviolet family of dyes. This dye is a tandem fluorochrome of BD Horizon BUV395 with an Ex Max of 348-nm and an acceptor dye with an Em Max at 661-nm. BD Horizon Brilliant BUV661 can be excited by the ultraviolet laser (355 nm) and detected with a 670/25 filter and a 630 nm LP. Due to cross laser excitation of this dye, there may be significant spillover into channels detecting APC-like emissions (eg, 670/25-nm filter).
Due to spectral differences between labeled cells and beads, using BD™ CompBeads can result in incorrect spillover values when used with BD Horizon BUV661 reagents. Therefore, the use of BD CompBeads or BD CompBeads Plus to determine spillover values for these reagents is not recommended. Different BUV661 reagents (eg, CD4 vs. CD45) can have slightly different fluorescence spillover therefore, it may also be necessary to use clone-specific compensation controls when using these reagents.
Development References (7)
-
Angelini DF, Zambello R, Galandrini R, et al. NKG2A inhibits NKG2C effector functions of γδ T cells: implications in health and disease.. J Leukoc Biol. 2011; 89(1):75-84. (Clone-specific: Flow cytometry). View Reference
-
Dulphy N, Haas P, Busson M, et al. An unusual CD56(bright) CD16(low) NK cell subset dominates the early posttransplant period following HLA-matched hematopoietic stem cell transplantation.. J Immunol. 2008; 181(3):2227-37. (Clone-specific: Flow cytometry). View Reference
-
Gumá M, Busch LK, Salazar-Fontana LI, et al. The CD94/NKG2C killer lectin-like receptor constitutes an alternative activation pathway for a subset of CD8+ T cells.. Eur J Immunol. 2005; 35(7):2071-80. (Biology). View Reference
-
Houchins JP, Yabe T, McSherry C, Bach FH. DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells.. J Exp Med. 1991; 173(4):1017-20. (Biology). View Reference
-
Lanier LL, Corliss B, Wu J, Phillips JH. Association of DAP12 with activating CD94/NKG2C NK cell receptors.. Immunity. 1998; 8(6):693-701. (Biology). View Reference
-
Picardi A, Mengarelli A, Marino M, et al. Up-regulation of activating and inhibitory NKG2 receptors in allogeneic and autologous hematopoietic stem cell grafts.. J Exp Clin Cancer Res. 2015; 34:98. (Clone-specific: Flow cytometry). View Reference
-
Warren HS. The Eighth Human Leucocyte Differentiation Antigen (HLDA8) Workshop: natural killer cell section report.. Cell Immunol. 236(1-2):17-20. (Clone-specific: Flow cytometry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.