Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
For optimal and reproducible results, BD Horizon Brilliant Stain Buffer should be used anytime two or more BD Horizon Brilliant dyes (including BD OptiBuild Brilliant reagents) are used in the same experiment. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. The BD Horizon Brilliant Stain Buffer was designed to minimize these interactions. More information can be found in the Technical Data Sheet of the BD Horizon Brilliant Stain Buffer (Cat. No. 563794).
Product Notices
- This antibody was developed for use in flow cytometry.
- The production process underwent stringent testing and validation to assure that it generates a high-quality conjugate with consistent performance and specific binding activity. However, verification testing has not been performed on all conjugate lots.
- Researchers should determine the optimal concentration of this reagent for their individual applications.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- BD Horizon Brilliant Stain Buffer is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,575,303; 8,354,239.
- BD Horizon Brilliant Ultraviolet 395 is covered by one or more of the following US patents: 8,158,444; 8,575,303; 8,354,239.
Companion Products
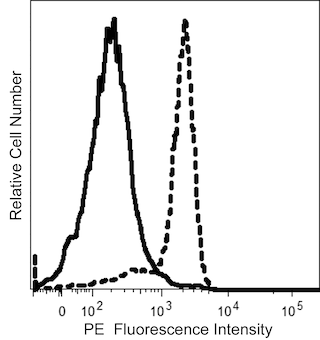




The GAP.A3 monoclonal antibody specifically recognizes the polymorphic HLA class I histocompatibility antigen, A-3 alpha chain (HLA-A3). This ~44 kDa human major histocompatibility complex (MHC) class I molecule noncovalently associates with the ~12 kDa monomorphic β2 microglobulin. Two major subtypes are encoded by HLA-A3, HLA-A3.1 and HLA-A3.2. HLA-A3 gene expression is more commonly detected in individuals from Europe and southern India. HLA-A3 is expressed on nearly all nucleated cells of HLA-A3-positive individuals. HLA-A3 functions in the presentation of antigens to CD8-positive T cells which may lead to the generation of HLA-A3-restricted cytotoxic T cells and memory T cells. When complexed with certain antigens, HLA-A3 can also bind to killer cell immunoglobulin-like receptors (KIR), such as KIR3DL2, and might regulate NK cell function.
Note: Since HLA-A3 expression varies between human populations, clone GAP.A3 staining can be donor-dependent. Based on in-house testing and current literature, individuals of European or Southern Indian descent more frequently express HLA-A3 than those of Asian descent. Data may differ between donors due to geographical variations of HLA-A3 expression.
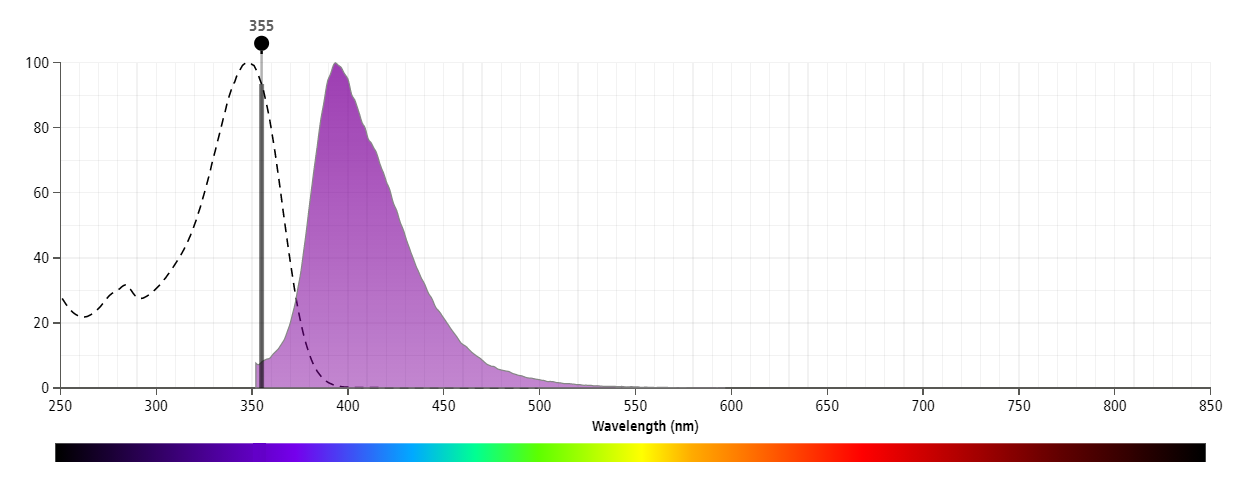
The antibody was conjugated to BD Horizon™ BUV395 which is part of the BD Horizon Brilliant™ Ultraviolet family of dyes. This dye has been exclusively developed by BD Biosciences to have minimal spillover into other detectors, making it an optimal choice for multicolor flow cytometry. With an Ex Max at 348 nm and an Em Max at 395 nm, BD Horizon BUV395 can be excited with a 355 nm laser and detected with a 379/28 filter.
Development References (7)
-
Allen TM, Altfeld M, Yu XG, et al. Selection, transmission, and reversion of an antigen-processing cytotoxic T-lymphocyte escape mutation in human immunodeficiency virus type 1 infection.. J Virol. 2004; 78(13):7069-78. View Reference
-
Berger AE, Davis JE, Cresswell P. Monoclonal antibody to HLA-A3.. Hybridoma. 1982; 1(2):87-90. View Reference
-
Bertrams HJ, Kuwert EK. Association of histocompatibility haplotype HLA-A3-B7 with multiple sclerosis.. J Immunol. 1976; 117(5 Pt.2):1906-12. View Reference
-
De Groot AS, Levitz L, Ardito MT, et al. Further progress on defining highly conserved immunogenic epitopes for a global HIV vaccine: HLA-A3-restricted GAIA vaccine epitopes.. Hum Vaccin Immunother. 2012; 8(7):987-1000. View Reference
-
Degioanni A, Darlu P, Raffoux C. Analysis of the French National Registry of unrelated bone marrow donors, using surnames as a tool for improving geographical localisation of HLA haplotypes.. Eur J Hum Genet. 2003; 11(10):794-801. View Reference
-
Walker EM, Walker SM. Effects of iron overload on the immune system.. Ann Clin Lab Sci. 2000; 30(4):354-65. View Reference
-
Zhang S, Liu J, Cheng H, et al. Structural basis of cross-allele presentation by HLA-A*0301 and HLA-A*1101 revealed by two HIV-derived peptide complexes.. Mol Immunol. 2011; 49(1-2):395-401. View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.