-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- Portugal (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
BD® CD4 (SK3) BV605
Clone SK3 (also known as Leu3a) (CE_IVD)

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
The antibody reagent is stable until the expiration date shown on the label when stored at 2° to 8°C. Do not use after the expiration date. Do not freeze the reagent or expose it to direct light during storage or incubation with cells. Keep the outside of the reagent vial dry.Do not use the reagent if you observe any change in appearance. Precipitation or discoloration indicates instability or deterioration.
Product Notices
- This reagent has been pre-diluted for use at the recommended Volume per Test. We typically use 1 × 10^6 cells in a 100-µl experimental sample (a test).
- Human donor specific background has been observed in relation to the presence of anti-polyethylene glycol (PEG) antibodies, developed as a result of certain vaccines containing PEG, including some COVID-19 vaccines. We recommend use of BD Horizon Brilliant™ Stain Buffer in your experiments to help mitigate potential background. For more information visit https://www.bdbiosciences.com/en-us/support/product-notices.
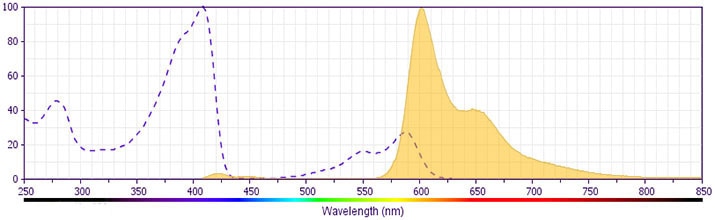
Development References (19)
-
Bernard A, Boumsell L, Hill C. Joint report of the first international workshop on human leucocyte differentiation antigens by the investigators of the participating laboratories: T2 protocol. In: Bernard A. A. Bernard .. et al., ed. Leucocyte typing : human leucocyte differentiation antigens detected by monoclonal antibodies : specification, classification, nomenclature = Typage leucocytaire : antigènes de différenciation leucocytaire humains révélés par les anticorps monoclonaux : "Rapports des études communes". Berlin New York: Springer-Verlag; 1984:25-60.
-
Centers for Disease Control and Prevention. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings. Available: https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html March 12, 2019. View Reference
-
Centers for Disease Control. Perspectives in disease prevention and health promotion update: universal precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other bloodborne pathogens in health-care settings. MMWR. 1988; 37:377-388.
-
Clinical and Laboratory Standards Institute. Clinical Flow Cytometric Analysis of Neoplastic Hematolymphoid Cells. In: CLSI. CLSI, ed. CLSI document H43-A2. Wayne, PA: Clinical and Laboratory Standards Institute; 2007:1-81. View Reference
-
Clinical and Laboratory Standards Institute. Collection of Diagnostic Venous Blood Specimens, 7th ed. In: CLSI. CLSI, ed. CLSI document GP41-A7. Wayne, PA: Clinical and Laboratory Standards Institute; 2017:1-85. View Reference
-
Clinical and Laboratory Standards Institute. Protection of Laboratory Workers from Occupationally Acquired Infections. In: CLSI. CLSI, ed. CLSI document M29-A4. Wayne, PA: Clinical and Laboratory Standards Institute; 2014:1-133. View Reference
-
Engleman EG, Warnke R, Fox RI, Dilley J, Benike CJ, Levy R. Studies of a human T lymphocyte antigen recognized by a monoclonal antibody.. Proc Natl Acad Sci USA. 1981; 78(3):1791-5. View Reference
-
Evans RL, Wall DW, Platsoucas CD, et al. Thymus-dependent membrane antigens in man: inhibition of cell-mediated lympholysis by monoclonal antibodies to TH2 antigen. Proc Natl Acad Sci U S A. 1981; 78(1):544-548. View Reference
-
Jackson AL, Warner NL. Rose NR, Friedman H, Fahey JL, ed. Manual of Clinical Laboratory Immunology 3rd ed. Washington, DC: American Society for Microbiology; 1986:226-235.
-
Kotzin BL, Benike CJ, Engleman EG. Induction of immunoglobulin-secreting cells in the allogeneic mixed leukocyte reaction: regulation by helper and suppressor lymphocyte subsets in man. J Immunol. 1981; 127(9):931-935. View Reference
-
Kroll MH. Evaluating interference caused by lipemia.. Clin Chem. 2004; 50(11):1968-9. View Reference
-
Ledbetter JA, Evans RL, Lipinski M, Cunningham-Rundles C, Good RA, Herzenberg LA. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981; 153(2):310-323. View Reference
-
Mady YH, Sayed NG, Zakaria MA, Sayed DM. Assessment of the use of 8-color flow cytometry in the diagnosis of acute leukemia. SECI Oncology. 2019; 7:8-20.
-
Nikolac N. Lipemia: causes, interference mechanisms, detection and management.. Biochem Med (Zagreb). 2014; 24(1):57-67. View Reference
-
Rothe G, Schmitz G. Consensus protocol for the flow cytometric immunophenotyping of hematopoietic malignancies. Working Group on Flow Cytometry and Image Analysis.. Leukemia. 1996; 10(5):877-95. View Reference
-
Sawada Y, Nakamura M, Kabashima-Kubo R, Shimauchi T, Kobayashi M, Tokura Y. Defective epidermal innate immunity and resultant superficial dermatophytosis in adult T-cell leukemia/lymphoma.. Clin Cancer Res. 2012; 18(14):3772-9. View Reference
-
Seegmiller AC, Kroft SH, Karandikar NJ, McKenna RW. Characterization of immunophenotypic aberrancies in 200 cases of B acute lymphoblastic leukemia.. Am J Clin Pathol. 2009; 132(6):940-9. View Reference
-
Stelzer GT, Marti G, Hurley A, McCoy PJ, Lovett EJ, Schwartz A. US-Canadian consensus recommendations on the immunophenotypic analysis of hematologic neoplasia by flow cytometry: standardization and validation of laboratory procedures. Cytometry. 1997; 30:214-230.
-
Uchida N, Otsuka T, Arima F, et al. Correlation of telomerase activity with development and progression of adult T-cell leukemia.. Leuk Res. 1999; 23(3):311-6. View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
![]() Documents are subject to revision without notice. Please verify you have the correct revision of the document, and always refer back to BD's eIFU website for the latest and most up to date information.
Documents are subject to revision without notice. Please verify you have the correct revision of the document, and always refer back to BD's eIFU website for the latest and most up to date information.