Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?

.png)

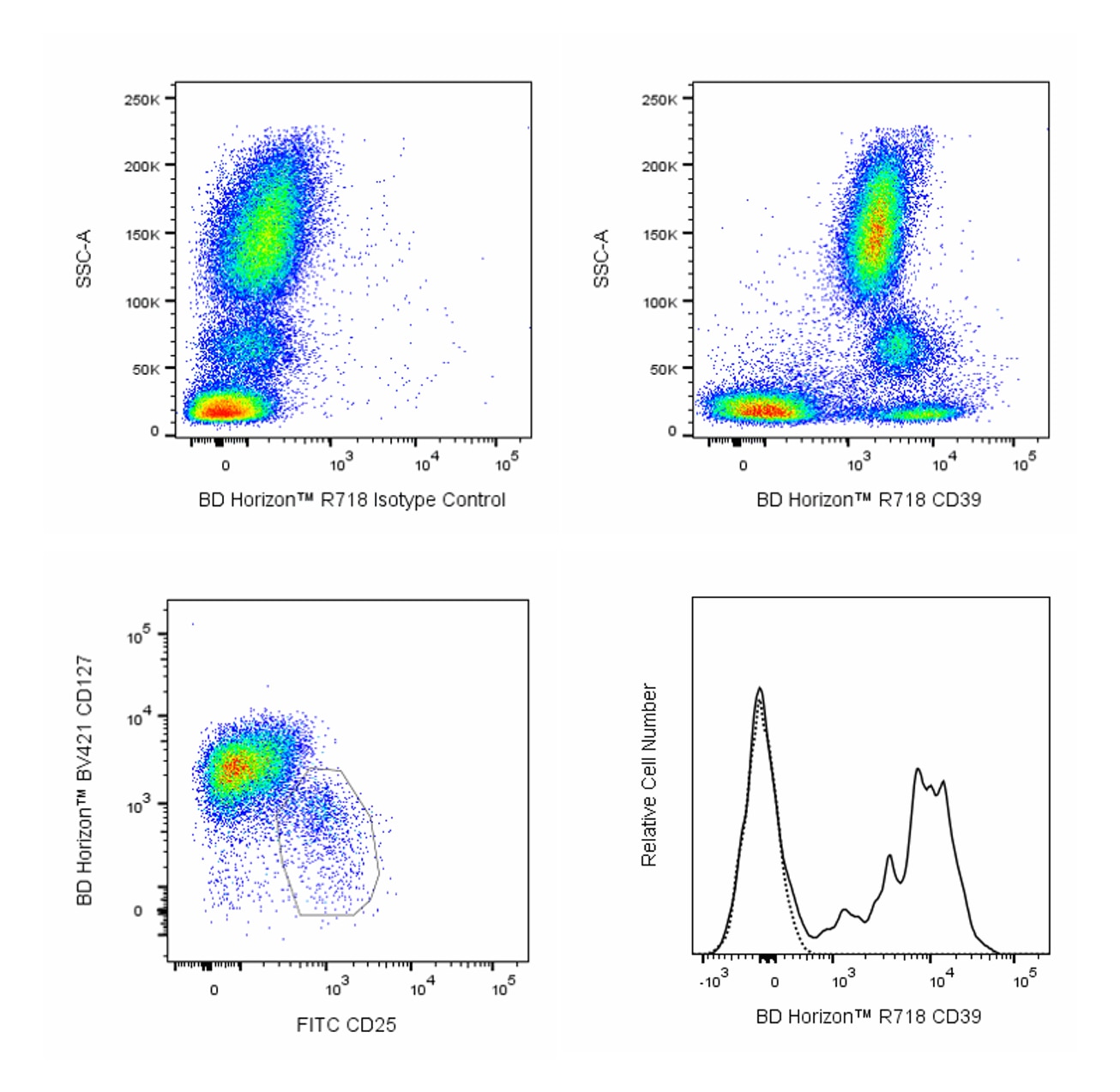
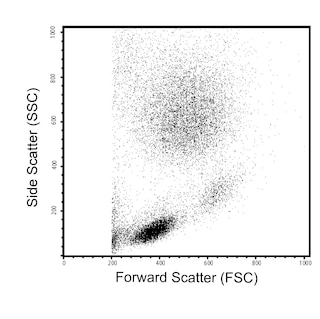
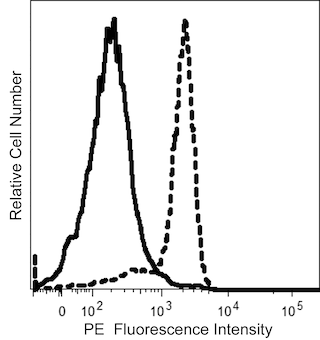
Flow cytometric analysis of CD39 expression on human peripheral blood leucocyte populations. Upper Plots: Whole blood was stained with either BD Horizon™ R718 Mouse IgG1, κ Isotype Control (Cat No. 566928; Left Plot) or BD Horizon™ R718 Mouse Anti-Human CD39 antibody (Cat No. 567674/567675; Right Plot). Erythrocytes were lysed with BD FACS™ Lysing Solution (Cat No. 349202). A bivariate pseudocolor density plot showing the correlated expression of CD39 (or Ig Isotype control staining) versus side light-scatter signals (SSC-A) was derived from gated events with the forward and side light-scatter characteristics of intact leucocyte populations. Lower Plots: Human peripheral blood mononuclear cells (PBMC) were preincubated with Human BD Fc Block™ (Cat. No. 564219/564220) and then stained with BD Horizon™ BUV395 Mouse Anti-Human CD4 (Cat. No. 564724), FITC Mouse Anti-Human CD25 (Cat. No. 555431/560990), BD Horizon™ BV421 Mouse Anti-Human CD127 (Cat No. 562436/562437) antibodies, and either BD Horizon™ R718 Mouse IgG1, κ Isotype Control (dashed line histogram) or BD Horizon™ R718 Mouse Anti-Human CD39 antibody (solid line histogram). DAPI (4',6-Diamidino-2-Phenylindole, Dihydrochloride) Solution (Cat. No. 564907) was added to cells right before analysis. Bivariate pseudocolor density plots showing the coexpressed levels of CD25 versus CD127 by viable (DAPI-negative) light scatter-gated CD4+ T cells [Left Plot] were further gated to reveal CD39 expression or Ig Isotype control staining [Right Plot] on CD4+CD25+CD127low T cells (ie, cells with a Regulatory T cell immunophenotype) as shown. Flow cytometric analysis was performed using a LSRFortessa™ X-20 Flow Cytometer System and FlowJo™ software.
.png)

BD Horizon™ R718 Mouse Anti-Human CD39
.png)
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
BD® CompBeads can be used as surrogates to assess fluorescence spillover (Compensation). When fluorochrome conjugated antibodies are bound to BD® CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cells and BD CompBeads to ensure that BD® CompBeads are appropriate for your specific cellular application.
Product Notices
- This reagent has been pre-diluted for use at the recommended Volume per Test. We typically use 1 × 10^6 cells in a 100-µl experimental sample (a test).
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- This product is provided under an Agreement between BIOTIUM and BD Biosciences. This product, and only in the amount purchased by buyer, may be used solely for buyer’s own internal research, in a manner consistent with the accompanying product literature. No other right to use, sell or otherwise transfer (a) this product, or (b) its components is hereby granted expressly, by implication or by estoppel. This product is for research use only. Diagnostic uses require a separate license from Biotium, Inc. For information on purchasing a license to this product including for purposes other than research, contact Biotium, Inc., 3159 Corporate Place, Hayward, CA 94545, Tel: (510) 265-1027. Fax: (510) 265-1352. Email: btinfo@biotium.com.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- Alexa Fluor™ is a trademark of Life Technologies Corporation.
Companion Products


.png?imwidth=320)

The A1 monoclonal antibody specifically recognizes human CD39 which is also known as Ecto-ATP diphosphohydrolase 1 (Ecto-ATPase 1 or Ecto-ATPDase 1), Ecto-apyrase or NTPDase 1. CD39 is a ~78 kDa integral membrane glycoprotein that is encoded by ENTPD1 (Ectonucleoside triphosphate diphosphohydrolase 1). CD39 contains two transmembrane domains, one having a N- and the other a C-terminal cytoplasmic tail, and a large extracellular domain that has the enzymatic site. CD39 is also known as Lymphoid cell activation antigen because its expression is induced upon activation of T and B cells. CD39 is variably expressed on some regulatory T cells, NK cells, granulocytes, monocytes, dendritic cells, Langerhans cells, endothelial cells, platelets, and neurons. CD39 is a member of the ectonucleoside triphosphate dihydrolases (E-NTPDases) family that is involved in the regulation of extracellular nucleotide catabolism by controlling the extracellular nucleoside triphosphate pool (NTP). It functions as an ectoenzyme that can hydrolyze both nucleoside triphosphates and diphosphates such as ATP and ADP and thereby suppress inflammation and regulate platelet activation as well as purinergic neurotransmission. The ectoenzymes CD39 and CD73 can act in tandem to enable regulatory T cells (Treg) to generate immunosuppressive adenosine and thereby regulate immune responses.
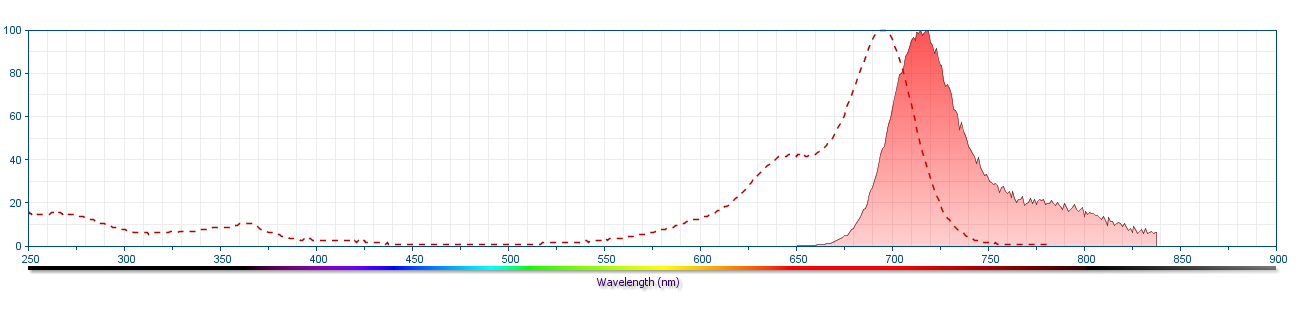
The antibody was conjugated to BD Horizon™ Red 718, which has been developed exclusively by for BD Biosciences as a better alternative to Alexa Fluor™ 700. BD Horizon™ Red 718 can be excited by the red laser (628 – 640 nm) and, with an Em Max around 718 nm, it can be detected using a 730/45 nm filter. Due to similar excitation and emission properties, we do not recommend using R718 in combination with APC-R700 or Alexa Fluor™ 700.
Development References (6)
-
Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets.. Immunol Rev. 2017; 276(1):121-144. (Biology). View Reference
-
Aversa GG, Suranyi MG, Waugh JA, Bishop AG, Hall BM. Detection of a late lymphocyte activation marker by A1, a new monoclonal antibody.. Transplant Proc. 1988; 20(1):49-52. (Immunogen: Flow cytometry). View Reference
-
Aversa GG, Waugh JA, Bishop GA, Hall BM. Use of monoclonal antibodies to study in vivo and in vitro-activated lymphocytes.. Transplant Proc. 1989; 21(1 Pt 1):349-50. (Clone-specific: Flow cytometry). View Reference
-
Gouttefangeas C, Mansur I, Bensussan A, Boumsell L. Biochemical analysis and epitope mapping of mAb defining CD39. In: Schlossman SF. Stuart F. Schlossman .. et al., ed. Leucocyte typing V : white cell differentiation antigens : proceedings of the fifth international workshop and conference held in Boston, USA, 3-7 November, 1993. Oxford: Oxford University Press; 1995:383-385.
-
Häusler SF, Del Barrio IM, Diessner J, et al. Anti-CD39 and anti-CD73 antibodies A1 and 7G2 improve targeted therapy in ovarian cancer by blocking adenosine-dependent immune evasion.. Am J Transl Res. 2014; 6(2):129-39. (Clone-specific: Flow cytometry, Functional assay, Inhibition). View Reference
-
Jones M, Mason DY. CD39 Workshop Panel report. In: Kishimoto T. Tadamitsu Kishimoto .. et al., ed. Leucocyte typing VI : white cell differentiation antigens : proceedings of the sixth international workshop and conference held in Kobe, Japan, 10-14 November 1996. New York: Garland Pub.; 1997:157-159.
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.