-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- BD OMICS-One™ WTA Next Assay
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?

Cell-Based Assays
Cell-based assays measure cellular processes, such as proliferation, viability, apoptosis and regulatory networks, and functional aspects of cells, such as phosphorylation and cytokine secretion. Using cell-based assays, entire pathways of interest can be interrogated, and multiple steps can be measured, yielding a functional readout of what is actually going on inside a cell. BD Biosciences offers several assays for intracellular cytokine signaling; cell cycle, cell viability and proliferation measurements; and assessing phosphorylation events.
Analysis of cell cycle, proliferation, viability and apoptosis
Cell growth, replication and division in eukaryotic cells occur according to a highly controlled series of events called the cell cycle. In adaptive immunity, specific T and B lymphocytes undergo clonal expansion (division, proliferation and differentiation) in response to foreign antigenic stimulation. Flow cytometry, immunofluorescence or immunohistochemistry can be used to quickly determine the cell cycle status, and by proxy, cellular response to stimulation.
Analysis of cell cycle and cellular DNA content
BD Biosciences offers a wide variety of reagents to study the cell cycle. Reagents include nucleic acid dyes such as propidium iodide (PI) and 7-aminoactinomycin D (7-AAD). In addition, the BD Cycletest™ Plus Reagent Kit includes PI and other reagents to degrade proteins and RNA to allow more precise DNA measurement. The samples are subsequently analyzed using flow cytometry to assess ploidy, identify abnormal DNA stemlines and estimate the DNA index (DI) and cell cycle phase distributions of stemlines. During the cell cycle phases, DNA levels change, facilitating the use of DNA dyes such as 7-AAD to generate characteristic cellular DNA content profiles.
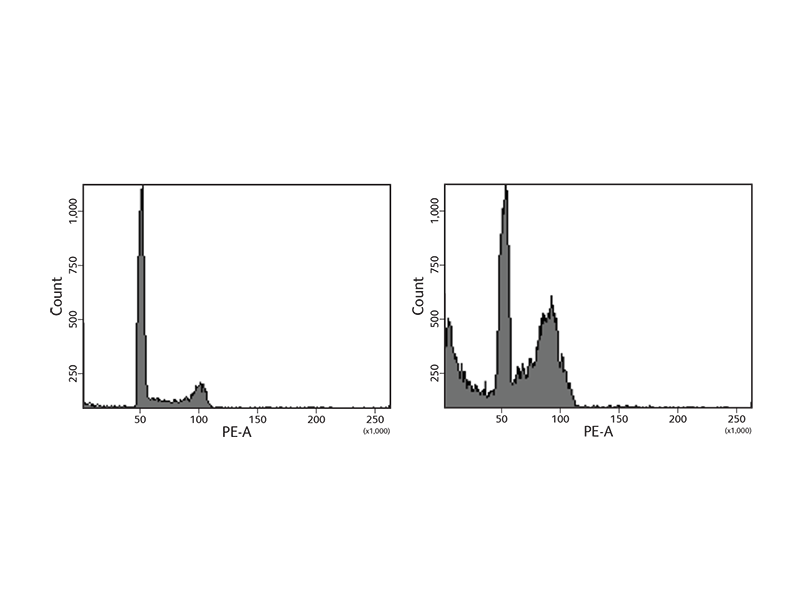
As cells go through the phases of the cell cycle, proteins such as histone H3 Ser28 become modified or change in expression. To facilitate DNA replication, the histone is modified, opening the chromatin to allow entry of replication machinery. To further support the study of the cell cycle, BD Biosciences carries antibodies to these proteins to use for imaging or flow cytometry applications.
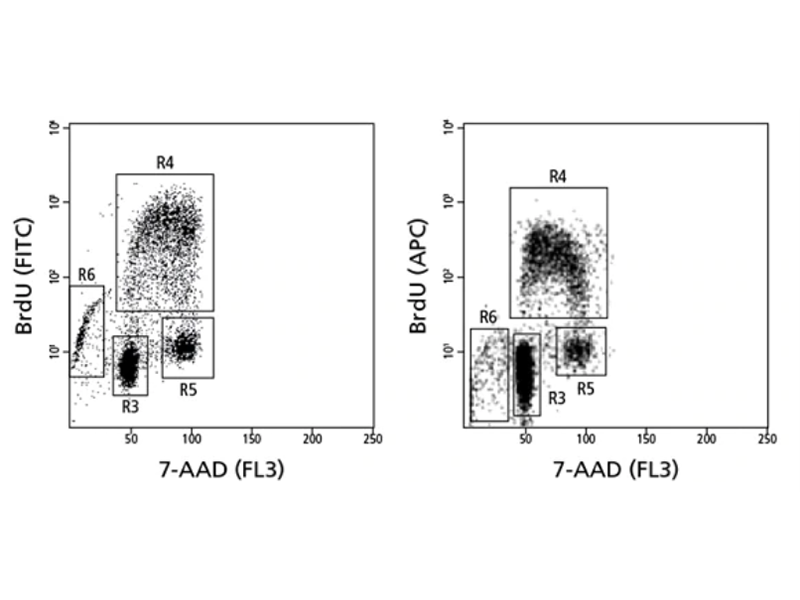
Cell cycle analysis of a population stained for incorporated bromodeoxyuridine (BrdU) and total DNA levels (7-AAD).
Find more information on the cell cycle and BD Biosciences solutions for cell cycle analysis.
Cell proliferation assays
Cell proliferation is an increase in the number of cells as a result of growth and division. The balance of cell proliferation and apoptosis is important for both development and normal tissue homeostasis. Multiple stimuli, such as cytokine treatment or receptor cross-linking with purified antibodies, can affect cell proliferation.
Measurement of cell proliferation with BrdU
BD Biosciences carries a series of antibodies and kits designed for the detection of proliferating cells by measurement of BrdU, an analog of the DNA precursor thymidine used to measure de novo DNA synthesis.
During the S phase of the cell cycle (DNA synthesis), BrdU is incorporated into newly synthesized DNA and can be readily detected by anti-BrdU specific antibodies. BD antibodies and kits designed for the detection of BrdU are available for both intracellular flow cytometry and immunohistochemistry and include BD Horizon™ Violet 450 (V450), BD Horizon Brilliant Violet™ 510 (BV510), BD Pharmingen™ PerCP-Cy5.5 and other formats.
In addition to DNA increases, levels of certain proteins also rise as a result of cell proliferation. For example, Ki67 is an antigen that is expressed in the nucleus of dividing cells. However, during the G0 phase of the cell cycle, it is not detected. Ki67 can be combined with other proliferation markers such as BrdU and VPD450 for added confidence. These markers can also be combined with cell surface and other types of markers to gain additional information about cell subsets and their signaling pathways.
Apoptosis and cell death analysis assays
The apoptotic process is characterized by certain morphological features. These include changes in the plasma membrane (such as loss of membrane symmetry and loss of membrane attachment), a condensation of the cytoplasm and nucleus, protein cleavage, and internucleosomal cleavage of DNA. In the final stages of the process, dying cells become fragmented into apoptotic bodies and consequently are eliminated by phagocytic cells without significant inflammatory damage to surrounding cells. BD Biosciences offers a full range of apoptosis detection tools and technologies for measuring indicators at different stages across the apoptotic process.
Annexin V—a key protein in apoptosis signaling
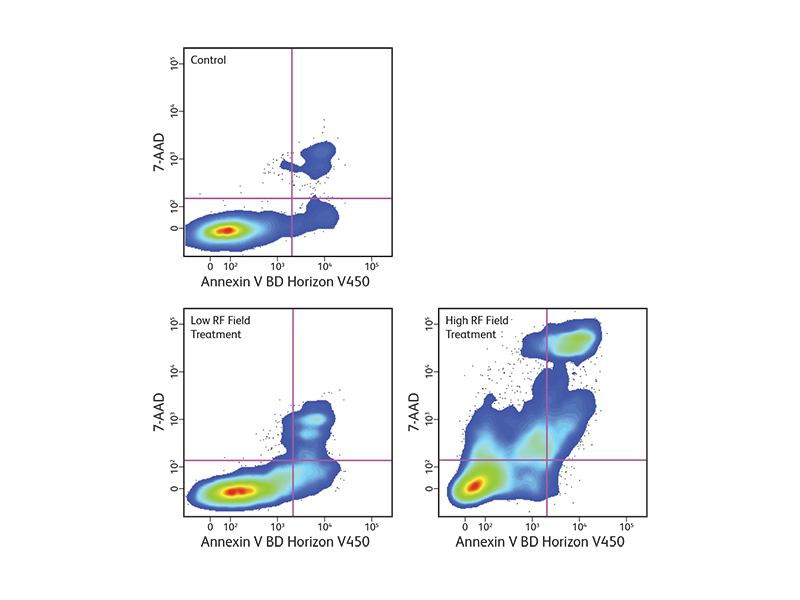
Changes in the plasma membrane are one of the characteristics of the apoptotic process detected in living cells. Apoptosis can be detected by the presence of phosphatidylserine (PS), which is normally located on the cytoplasmic face of the plasma membrane. During apoptosis, PS translocates to the outer leaflet of the plasma membrane and can be detected by flow cytometry and cell imaging through binding to fluorochrome-labeled annexin V when calcium is present.
BD Biosciences offers annexin V in several common formats, including FITC, PE, BD Horizon™ BV421 for the violet laser, BD Horizon Brilliant™ UV 395 (BUV395) for the ultraviolet laser, and more. With the addition of these new formats, more complex assays can be developed to look at apoptosis within heterogeneous cell subsets.
Since intracellular annexin V is also exposed if the plasma membrane is compromised, a membrane-impermeant dye such as 7-AAD is commonly used to distinguish between apoptotic and dead cells to exclude the dead cells. The populations of cells that are stained with annexin V only represent the apoptotic cell populations.
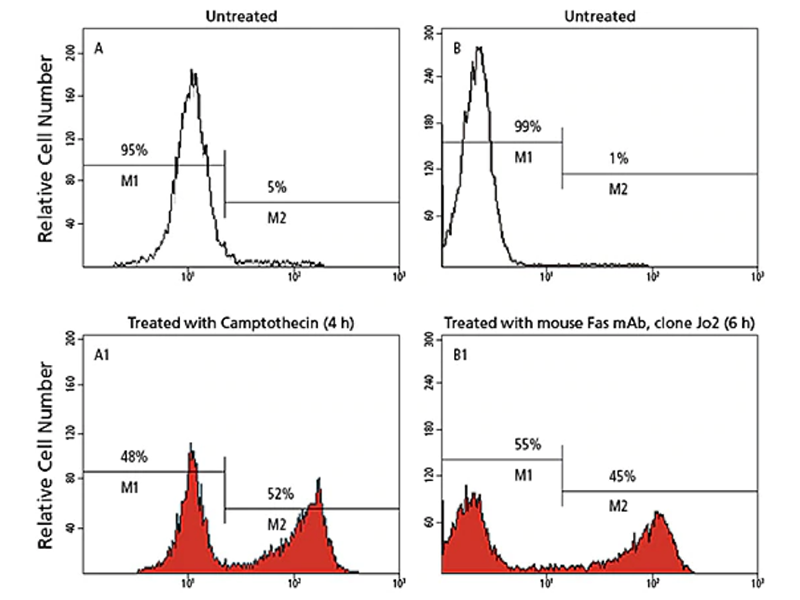
Measurement of cleaved caspases and PARP
One of the most consistently observed characteristics of apoptosis is the activation of a series of cytosolic proteases, called caspases, which are activated upon cleavage at aspartate residues during the earliest stages of apoptosis. Active caspases can then cleave many proteins, including poly-ADP ribose polymerase (PARP), other caspases and other protein substrates en masse. This leads to the loss of cellular structure and function and ultimately results in cell death. In particular, caspase-9, -8 and -3 have been implicated in apoptosis: caspase-9 in the mitochondrial pathway, caspase-8 in the Fas/CD95 pathway, and caspase-3 more downstream, activated by multiple pathways.
BD Biosciences carries a variety of reagents to measure caspases, particularly caspase-3. They include antibodies directed exclusively against the active form of the caspase. These antibodies are available in a variety of formats and can be used for flow cytometry, imaging, ELISA and western blotting.
Caspase-3 cleavage/inhibition reactions
Active caspase-3 binds to the fluorogenic Ac-DEVD-AMC substrate and cleaves it between asparatic acid (D) and AMC, releasing the fluorescent AMC. AMC fluorescence is quantified by UV spectrofluorometry. The Ac-DEVD-CHO aldehyde inhibitor binds strongly to the caspase-3 active site and blocks substrate binding. Hence, Ac-DEVD-AMC is not cleaved, and fluorescence is not emitted
BD Biosciences offers several protocols for analyzing cell cycle, proliferation, cell death and apoptosis.
Phosphoprotein analysis
Phosphorylation of tyrosine, serine and threonine residues is critical for the control of protein activity involved in various cellular events. An assortment of kinases and phosphatases regulate intracellular protein phosphorylation in many different cell signaling pathways, such as T and B cell signaling, those regulating apoptosis, growth and cell cycle control, plus those involved with cytokine, chemokine, and stress responses. Historically, phosphoprotein detection has been performed using techniques such as radiometric kinase assays and phosphoamino acid labeling. However, the advent of phospho-specific antibodies has facilitated the use of more straightforward techniques such as western blotting, immunoprecipitation and immunofluorescence microscopy. These techniques, however, have several shortcomings in that they require a relatively large amount of sample, are time consuming, do not produce truly quantitative results, and are not conducive to multiparameter analysis.
Phosphoprotein analysis combines phospho-specific antibodies with the power of flow cytometry to enhance phosphoprotein analysis. Flow cytometry requires only a small sample size and is ideal for performing rapid, quantitative, multiparameter analyses of single cells and distinct cell subpopulations. The BD Phosflow™ Reagents enable intracellular phosphoprotein analysis of single cells or subpopulations using flow cytometry.
High-content cell imaging enables researchers to visualize protein phosphorylation in its microenvironment and to study protein-protein interaction and translocation events simultaneously.
Detecting transient phosphorylation events: BD Phosflow™ Reagent technology
Innovative BD Phosflow™ Reagent technology was the first complete flow cytometry solution to reveal intracellular data on basal and induced protein phosphorylation events in both cell lines and primary cells. The BD Phosflow™ Reagent approach is especially informative for studying T cells, in which phosphorylation of signaling proteins leads to the expression of particular T cell phenotypes.
BD Biosciences BD Phosflow™ Reagent Protocols for use with different sample types.
BD Cytofix/Cytoperm™ Reagent method
The BD Cytofix/Cytoperm™ Reagent method and related reagents address the wide-ranging experimental needs of basic research. With products for a broad selection of cytokines and multiple species, BD Cytofix/Cytoperm™ Reagents offer maximum flexibility in intracellular cytokine staining. BD Biosciences offers several solutions for measuring intracellular cytokines through intracellular flow cytometry.
BD FastImmune™ System
The BD FastImmune™ System is designed to meet the needs of applied research on human samples. With a focus on complete systems, this approach is well suited for research studies monitoring immune status during disease, or immune responses to vaccine candidates. The BD FastImmune™ CD4 Intracellular Cytokine Detection Kit Anti-Hu-TNF-α/CD69/CD4/CD3 is designed for the detection of intracellular cytokines and the activation marker CD69 in antigen-activated CD4+ T lymphocytes in whole blood. Applications include studies of T cell responses to antigens, such as herpes viruses, HIV and tumor antigens. BD Biosciences offers several solutions for measuring intracellular cytokines through intracellular flow cytometry.
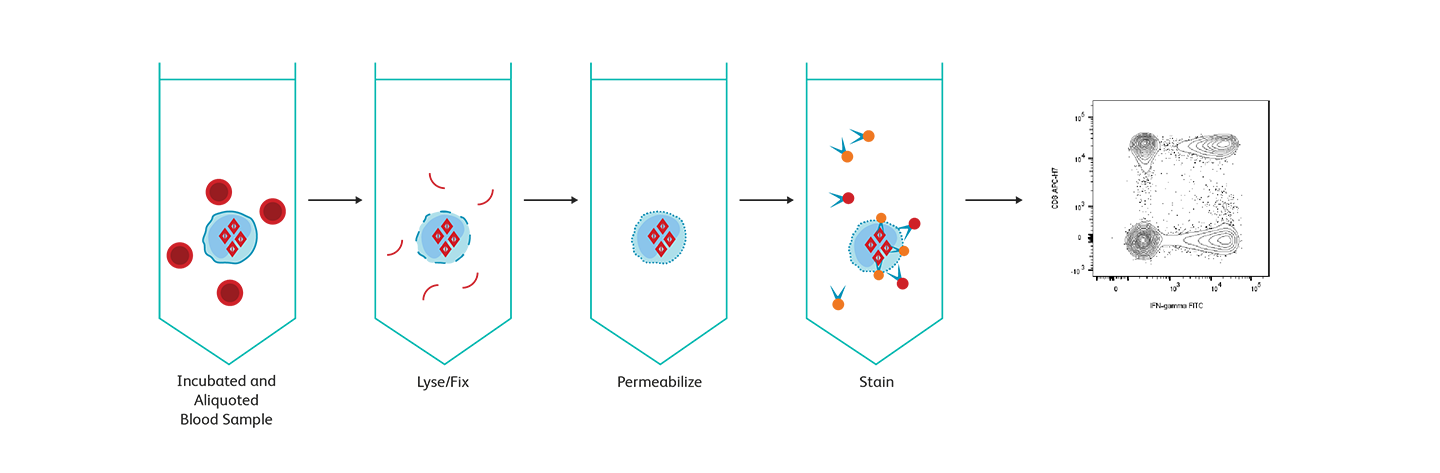
Analysis of intracellular cytokines
For intracellular protein detection, cells must be fixed and permeabilized to allow a fluorescent antibody to enter and detect the target protein of interest. Different antigens have different sensitivities to and requirements for fixation and permeabilization, entailing additional optimization of protocols. To detect cytokines, which are secreted proteins, protein transport inhibitors are required to trap proteins inside the cells. BD Biosciences offers two widely used assay systems for intracellular cytokine detection: BD Cytofix/Cytoperm™ Reagents and the BD FastImmune™ Cytokine System. Both systems offer researchers the ease and confidence of using reagents and protocols that are optimized for use together and incorporate the high standard of quality and reproducibility that BD flow cytometry products are known for. BD Phosflow™ Reagents help you use multicolor flow cytometry to measure the level of phosphorylated proteins involved in T cell signaling and combine the data with subset identification.
Analysis of secreted cytokines
For detection of secreted cytokines within a sample, BD offers multiple assays. BD® Cytometric Bead Array (CBA) technology allows quantitation of multiple soluble cytokines simultaneously, while BD OptEIA™ ELISA Reagents are designed for quantitation of single cytokines. BD® ELISPOT Reagents enable determination of the frequency of cytokine-producing cells, and BD® In Vivo Capture Assays allow quantitation by directly capturing cytokines in vivo.
BD Biosciences offers several protocols for ELISAs and other immunoassays.
Biological assays for chemokines and chemokine receptors
The chemotaxis assay and the calcium mobilization assay are used to characterize the biological activity of chemokines and their receptors.
Chemotaxis assay
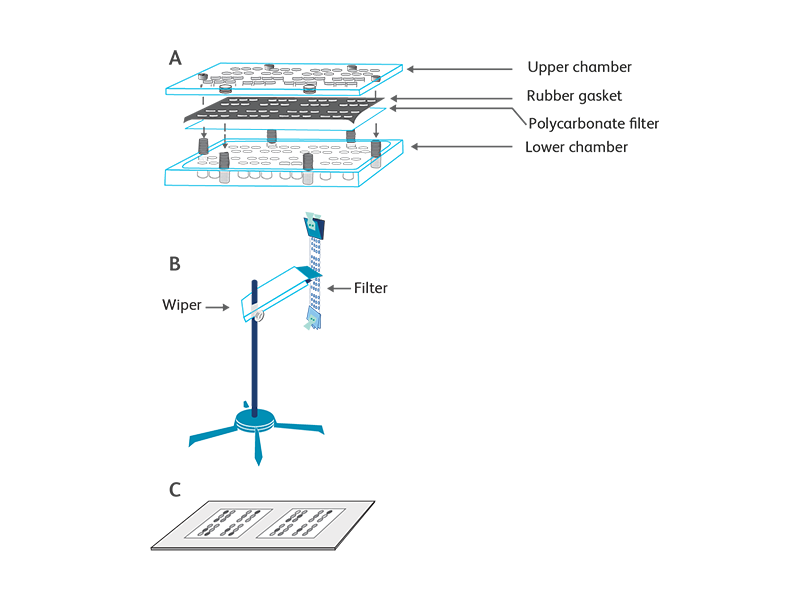
The chemotactic assay is based on the directional migration of target cells in response to chemokine gradients. The apparatus used for most assays is the Boyden chamber developed in the 1960s.1 Modern chemotaxis assays have adapted several modifications such as precoating the membranes with extracellular matrix proteins (e.g., collagen, fibronectin) or endothelial cell monolayers to mimic in vivo environments. The chemotaxis assay we employ at BD Biosciences utilizes a 48-well chemotaxis chamber (Neuro Probe, Cabin John, MD).2
Another modified chemotaxis assay that enumerates migrated cells by measurement of lactate dehydrogenase (LDH) upon cell lysis is also used to determine the biological activity of chemokines. Briefly, transwell inserts with 3- or 5-μm pore-size membranes are suspended in the wells of a 24-well plate containing controls and chemokines. The target cells are added into the transwell inserts. After incubation, the number of migrated cells is determined by LDH assay.3 The amount of released LDH is proportional to the enzymatic conversion of a tetrazolium salt (INT) into a red formazan that can be easily measured at a 490-nm wavelength of light using an ELISA plate reader.
Calcium mobilization assay
Transient increases in cytosolic free Ca2+ concentration ([Ca2+]i) can provide an indication of cellular activation for many ligand transmembrane receptor systems that are involved in cell signaling. For this reason, assays that measure the levels of transient [Ca2+]i flux caused by ligands, or that are prevented by antibodies directed against activating ligands or their receptors, can be used to determine the levels and specific activities of these biologically-active molecules.
Calcium ions play a unique role in intracellular signaling and are considered to be an important second messenger for cellular signaling pathways. Chemokines, anaphylatoxins and other inflammatory mediators may trigger calcium mobilization responses upon binding to their cellular receptors. In these cases, receptor-ligand interactions activate the guanine nucleotide binding proteins located on the inside of the membrane. Consequently, heterotrimeric G proteins activate phospholipase C to cleave phosphatidyl inositol 4,5-bisphosphate, releasing diacylglycerol and inositol triphosphate. Inositol triphosphate causes the release of Ca2+ from intracellular stores, while diacylglycerol and the increased cytosolic Ca2+ levels have been implicated in the activation of protein kinase C inside the cell. Increased phosphorylation events have in turn been related to oxidant production and secretory function by these activated cells.
There are a wide variety of available fluorescent indicator dyes, such as Indo-1 and Fura-2, which change their fluorescent properties after complexing with Ca2+. For example, when using a spectrofluorometer with an excitation light wavelength set at ~358 nm, the fluorescence emission maximum of Indo-1 shifts from ~485 nm in Ca2+-free medium to ~405 nm when the dye is saturated with Ca2+. The ratio of fluorescence of the Ca2+-bound dye and the Ca2+-free dye can be used to determine [Ca2+]i. The cell-permeable acetoxymethyl (AM) esters of these dyes can be passively loaded into cells, where they are cleaved to cell-impermeable products by intracellular esterases. To perform a calcium mobilization assay, the target cells are loaded with Indo-1, placed in a temperature-controlled (37 °C) stirred cuvette inside the spectrofluorometer, and excited at a 358-nm wavelength of light. After determining the baseline emission at 405 and 485 nm, the stimulant (chemokine, anaphylatoxin or other inflammatory mediator) is rapidly injected into the cell suspension. Emitted fluorescent light signals are continuously monitored and recorded for the next 120–300 seconds.
The level of cytosolic free Ca2+, as reflected by the ratio of emissions (E405/E485), increases rapidly if the ligand is stimulatory (i.e., causes its receptor to transduce signals inside the cell that result in the mobilization of Ca2+ from intracellular stores into the cytosol). This response is followed by a decrease of the [Ca2+]i back to baseline levels. The amplitude of the transient increase of cytosolic free Ca2+ is dependent on the stimulatory ligand concentration used to activate the target cells allowing for the determination of an ED50.
Other methods
In addition to the methods described above, several methods have been used to determine the biological activities of certain chemokines. These include CD11b/CD18 upregulation assays for both CC and CXC chemokines; neutrophil elastase or β-glucuronidase release assays and neutrophil oxidative burst assays for CXC chemokines; hematopoietic colony formation assays for MIP-1α and MIP-1β; and histamine release assays for CC chemokines.
References
- Boyden S. The chemotactic effect of mixture of antibody and antigen on polymorphonuclear leukocytes. J Exp Med. 1962;115(3):453-466. doi: 10.1084/jem.115.3.453
- Leonard EJ, Sylvester I, Yoshimura T. Measurement of human neutrophil attractant protein-1 (NAP-1; IL-8). In Coligan JE, Kruisbeek M, Margulies DH, Shevach EM, Strober W, eds. Current Protocols in Immunology. Greene Publishing Associates and Wiley-Interscience; 1992.
- Nachlas MM, Margulies SI, Goldberg JD, Seligman AM. The determination of lactic dehydrogenase with a tetrazolium salt. Anal Biochem. 1960;1:317-326. doi: 10.1016/0003-2697(60)90029-4
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Cy is a trademark of Global Life Sciences Solutions Germany GmbH or an affiliate doing business as Cytiva.