Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
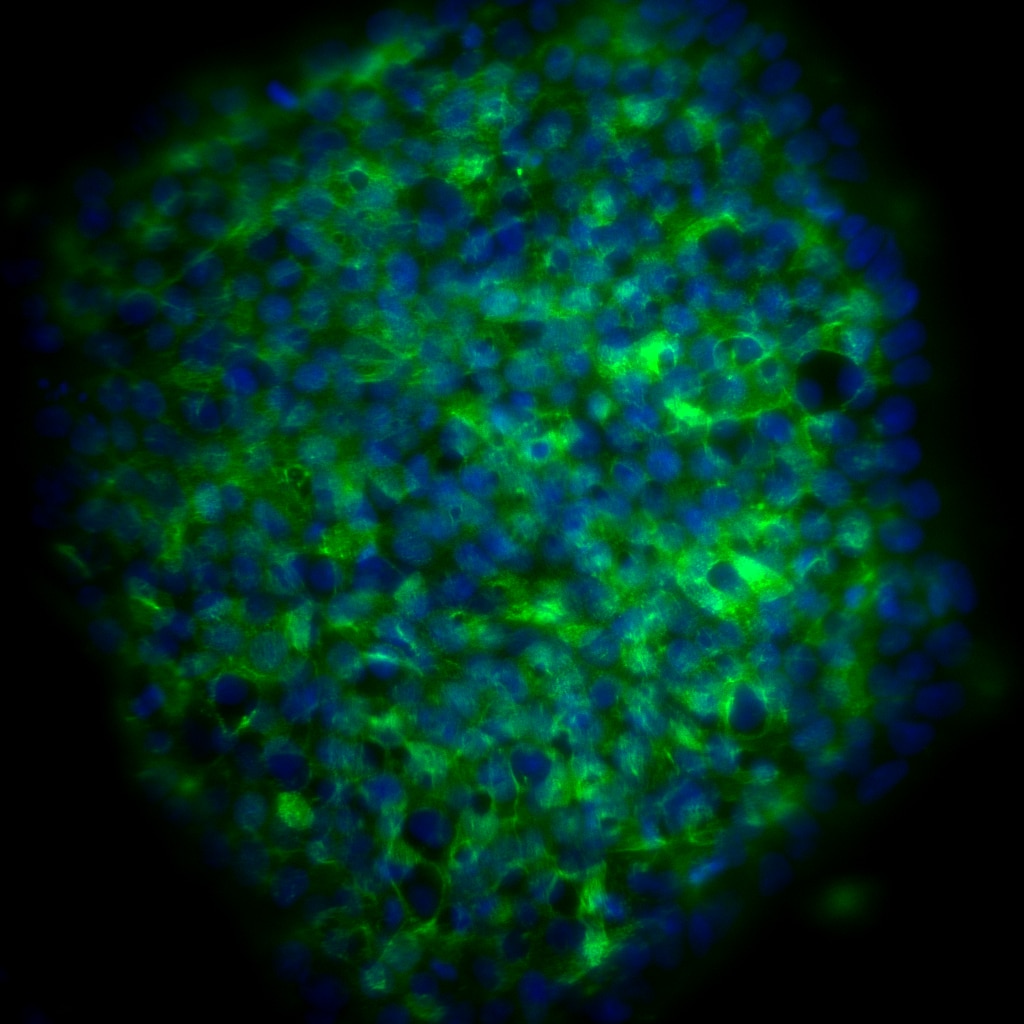





Immunofluorescent staining for Podocalyxin Associated Keratan Sulfate on human embryonic stem (ES) cells. H9 human ES cells (WiCell, Madison, WI) passage 60 grown in mTESR™1 medium (StemCell Technologies) were fixed with BD Cytofix™ Fixation Buffer (Cat. No. 554655). Cells were stained with Purified Mouse Anti-Human Podocalyxin Associated Keratan Sulfate monoclonal antibody (pseudo-colored green) at 20 μg/mL. The second-step reagent was Alexa Fluor® 488 Goat Anti-Mouse Ig (Life Technologies), and cell nuclei were stained with BD Pharmingen™ DAPI Solution (Cat. No. 564907) (pseudo-colored blue). The images were captured on a BD Pathway™ 435 Cell Analyzer and merged using BD Attovision™ software.

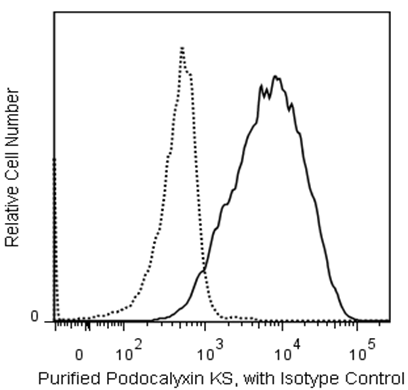
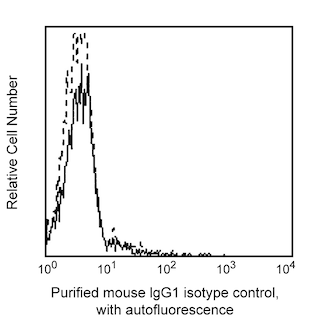
Flow cytometric analysis of Podocalyxin Associated Keratan Sulfate on human embryonic stem (ES) cells. H9 human embryonic stem (ES) cells (WiCell, Madison, WI) passage 59 grown in mTESR™1 medium (StemCell Technologies) were dissociated with BD™ Accutase™ Cell Detachment Solution (Cat. No. 561527) and stained with Purified Mouse Anti-Human Podocalyxin Associated Keratan Sulfate (solid line histogram) or Purified Mouse IgG1 κ Isotype Control (Cat. No. 554121, dashed line histogram). The second-step reagent was PE Goat Anti-Mouse Ig (Cat. No. 550589). The fluorescence histograms were derived from gated events with the forward and side light-scatter characteristics of viable H9 cells, respectively. Flow cytometry was performed on a BD FACSCanto™ II flow cytometry system.

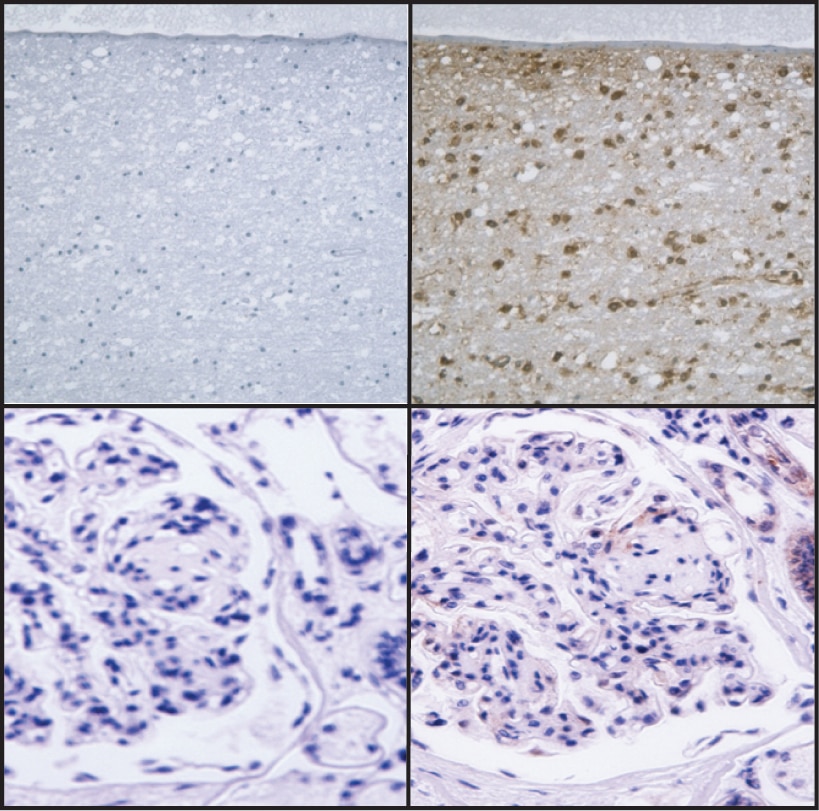
Immunohistochemical staining for Podocalyxin Associated Keratan Sulfate on human brain (top row) and kidney (bottom row). Following antigen retrieval with BD Retrievagen A Buffer (Cat. No. 550524), the formalin-fixed paraffin-embedded sections were stained with either Purified Mouse IgG1 κ Isotype Control (Cat. No.550878; Left Column) or Purified Mouse Anti-Human Podocalyxin Associated Keratan Sulfate (Right Column). A three-step staining procedure that employs a Biotin Goat Anti-Mouse Immunoglobulin (Cat. No. 550337), Streptavidin-Horseradish Peroxidase (HRP) (Cat. No.550946), and the DAB Substrate Kit (Cat. No. 550880) was used to develop the primary staining reagents. Original magnification: 20×.


BD Pharmingen™ Purified Mouse Anti-Human Podocalyxin Associated Keratan Sulfate

BD Pharmingen™ Purified Mouse Anti-Human Podocalyxin Associated Keratan Sulfate

BD Pharmingen™ Purified Mouse Anti-Human Podocalyxin Associated Keratan Sulfate

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- mTESR™1 is a trademark of StemCell Technologies.
- Alexa Fluor® is a registered trademark of Molecular Probes, Inc., Eugene, OR.
- Accutase is a registered trademark of Innovative Cell Technologies, Inc.
- Sodium azide is a reversible inhibitor of oxidative metabolism; therefore, antibody preparations containing this preservative agent must not be used in cell cultures nor injected into animals. Sodium azide may be removed by washing stained cells or plate-bound antibody or dialyzing soluble antibody in sodium azide-free buffer. Since endotoxin may also affect the results of functional studies, we recommend the NA/LE (No Azide/Low Endotoxin) antibody format, if available, for in vitro and in vivo use.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- An isotype control should be used at the same concentration as the antibody of interest.
Companion Products




The epitope recognized by the R-10G monoclonal antibody is a keratan sulfate that lacks oversulfated structures and is expressed on human pluripotent stem cells. Podocalyxin, a sialomucin playing a key role in cell adhesion and migration in kidney podocytes, hematopoietic progenitors and vascular cells, was identified as the carrier protein of the R-10G epitope. The R-10G monoclonal antibody specifically binds to human induced pluripotent stem cells (hiPSC) and human embryonic stem cells (hESC) and is expressed heterogeneously on pluripotent stem cells. When the R-10G antibody was used to stain an array of human tissues, significant reactivity was found in only adult brain and cerebellum. Unlike conventional pluripotent stem cell markers that are expressed both on stem cells and embryonal carcinoma cells, the R-10G epitope is not expressed on embryonal carcinoma cells and hence is a unique marker of hiPSC and hESC.
Purified Mouse Anti-Human Podocalyxin Associated Keratan Sulfate (clone R-10G; Cat. No. 565518) is routinely tested via Western Blot using human brain tissue lysate (Novus Bio; Cat. No. NB820-59177). Expected molecular weight is >200 kDa.
Development References (6)
-
Doyonnas R, Kershaw DB, Duhme C, et al. Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J Exp Med. 2001; 194(1):13-27. (Biology). View Reference
-
Kawabe K, Tateyama D, Toyoda H et al. A novel antibody for human induced pluripotent stem cells and embryonic stem cells recognizes a type of keratan sulfate lacking oversulfated structures. Glycobiology. 2013; 23(3):322-336. (Immunogen: ELISA, Immunofluorescence, Immunohistochemistry, Western blot). View Reference
-
Kelley TW, Huntsman D, McNagny KM, Roskelley CD, Hsi ED. Podocalyxin: a marker of blasts in acute leukemia. Am J Clin Pathol. 2005; 124(1):134-142. (Biology). View Reference
-
Kerjaschki D, Sharkey DJ, Farquhar MG. Identification and characterization of podocalyxin--the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol. 1984; 98(4):1591-1596. (Biology). View Reference
-
McNagny KM, Pettersson I, Rossi F, et al. Thrombomucin, a novel cell surface protein that defines thrombocytes and multipotent hematopoietic progenitors. J Cell Biol. 1997; 138(6):1395-1407. (Biology). View Reference
-
Somasiri A, Nielsen JS, Makretsov N, et al. Overexpression of the anti-adhesin podocalyxin is an independent predictor of breast cancer progression. Cancer Res. 2004; 64(15):5068-5073. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.