Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?




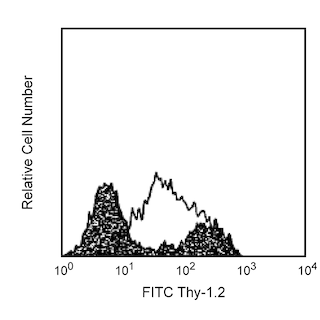
Flow cytometric analysis for IL-4 in activated mouse splenocytes. Mouse Intracellular Cytokine-2 positive control cells (MiCK-2) offered by BD Biosciences as MN 554653, are activated mouse splenocytes prepared in the presence of a protein transport inhibitor. Fixed and permeabilized MiCK-2 cells were stained either with a PerCP-Cy™5.5 Rat IgG1, κ isotype control (left panel) or with the PerCP-Cy™5.5 Rat Anti-Mouse IL-4 antibody (right panel). Dot plots were derived from gated events based on light scattering characteristics for lymphocytes. Flow cytometry was performed on a BD LSR™ II flow cytometry system.


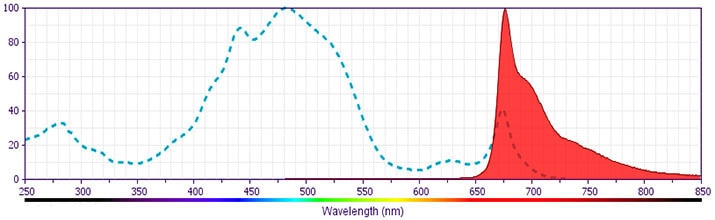
BD Pharmingen™ PerCP-Cy™5.5 Rat Anti-Mouse IL-4

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
Flow cytometry: The 11B11 antibody is useful for immunofluorescent staining and flow cytometric analysis to identify and enumerate IL-4 producing cells within mixed cell populations. A useful control investigators may consider using for demonstrating specificity of staining, is to pre-block with one of the following reagents: (1) recombinant mouse IL-4 (Cat. No. 550067) or (2) unlabeled 11B11 antibody (Cat. No. 554434), prior to staining.
Cell Preparation: Investigators not wishing to utilize MiCK-2 cells may alternatively stimulate mouse splenocyte enriched CD4+ cells (e.g C57BL/6) with 10 µg/ml plate-bound NA/LE hamster anti-mouse CD3e antibody (clone 145-2C11; Cat. No. 553057) and 2 µg/ml soluble NA/LE hamster anti-mouse CD28 (clone 37.51; Cat. No. 553294) antibody in the presence of 10 ng/ml recombinant mouse IL-2 (Cat. No. 550069) and 20 ng/ml recombinant mouse IL-4 (Cat. No. 550067) for 2 days followed by additional cell expansion with recombinant IL-2 and IL-4 for an additional 3 days. Following expansion, cells may be activated with the Leukocyte Activation Cocktail (Cat. No. 550583) or alternatively, with a 4-6 hr treatment with PMA (5 ng/mL, Sigma-Aldrich Cat. No. P-8139) and ionomycin (500 ng/mL, Sigma-Aldrich Cat. No. I-0634) in the presence of 1 µg/mL Brefeldin A (BD GolgiPlug™ MN 555029). Investigators are advised to fix and permeabilize the cells prior to staining.
BD® CompBeads can be used as surrogates to assess fluorescence spillover (compensation). When fluorochrome conjugated antibodies are bound to BD® CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cell and BD® CompBeads to ensure that BD® CompBeads are appropriate for your specific cellular application.
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- An isotype control should be used at the same concentration as the antibody of interest.
- Please observe the following precautions: Absorption of visible light can significantly alter the energy transfer occurring in any tandem fluorochrome conjugate; therefore, we recommend that special precautions be taken (such as wrapping vials, tubes, or racks in aluminum foil) to prevent exposure of conjugated reagents, including cells stained with those reagents, to room illumination.
- PerCP-Cy5.5 is optimized for use with a single argon ion laser emitting 488-nm light. Because of the broad absorption spectrum of the tandem fluorochrome, extra care must be taken when using dual-laser cytometers, which may directly excite both PerCP and Cy5.5™. We recommend the use of cross-beam compensation during data acquisition or software compensation during data analysis.
- PerCP-Cy5.5–labelled antibodies can be used with FITC- and R-PE–labelled reagents in single-laser flow cytometers with no significant spectral overlap of PerCP-Cy5.5, FITC, and R-PE fluorescence.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- Cy is a trademark of Global Life Sciences Solutions Germany GmbH or an affiliate doing business as Cytiva.
Companion Products


Interleukin-4 (IL-4) is a pleiotropic cytokine that has many roles, such as inducing the differentiation of naïve helper T cells (Th0 cells) to Th2 cells, stimulating activated B-cell and T-cell proliferation, and promoting immunoglobulin class switching to IgG1 and IgE in mouse B-cells. IL-4 is expressed by CD4 T-cells, mast cells, basophils and eosinophils. IL-4 was previously known as B-Cell Differentiation Factor (BCDF) or B-cell Stimulatory Factor (BSF1). The 11B11 monoclonal antibody specifically binds to mouse IL-4. The immunogen used to generate the 11B11 hybridoma was partially purified mouse IL-4 prepared from the supernatant of Phorbol 12-Myristate 13-Acetate (PMA)-stimulated EL-4 cells. The 11B11 antibody is reportedly a neutralizing antibody.
Development References (17)
-
Assenmacher M, Schmitz J, Radbruch A. Flow cytometric determination of cytokines in activated murine T helper lymphocytes: expression of interleukin-10 in interferon-gamma and in interleukin-4-expressing cells. Eur J Immunol. 1994; 24(5):1097-1101. (Biology). View Reference
-
Bogen SA, Fogelman I, Abbas AK. Analysis of IL-2, IL-4, and IFN-gamma-producing cells in situ during immune responses to protein antigens. J Immunol. 1993; 150(10):4197-4205. (Biology). View Reference
-
Chatelain R, Varkila K, Coffman RL. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992; 148(4):1182-1187. (Biology). View Reference
-
Finkelman FD, Madden KB, Morris SC, et al. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol. 1993; 151(3):1235-1244. (Biology). View Reference
-
Fujihashi K, McGhee JR, Beagley KW, et al. Cytokine-specific ELISPOT assay. Single cell analysis of IL-2, IL-4 and IL-6 producing cells. J Immunol Methods. 1993; 160(2):181-189. (Biology). View Reference
-
Gillis S, Ferm MM, Ou W, Smith KA. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978; 120(6):2027-2032. (Biology). View Reference
-
Haak-Frendscho M, Brown JF, Iizawa Y, Wagner RD, Czuprynski CJ. Administration of anti-IL-4 monoclonal antibody 11B11 increases the resistance of mice to Listeria monocytogenes infection. J Immunol. 1992; 148(12):3978-3985. (Biology). View Reference
-
Helms T, Boehm BO, Asaad RJ, Trezza RP, Lehmann PV, Tary-Lehmann M. Direct visualization of cytokine-producing recall antigen-specific CD4 memory T cells in healthy individuals and HIV patients. J Immunol. 2000; 164(7):3723-3732. (Biology). View Reference
-
Klinman D and Nutman T. ELISPOT assay to detect cytokine-secreting murine and human cells. In: Coligan J, Kruisbeek A, Margulies D, Shevach E, Strober W, ed. Current Protocols in Immunology. 1994:6-19.
-
Lindqvist C, Lundstrom H, Oker-Blom C, Akerman KE. Enhanced IL-4-mediated D10.G4.1 proliferation with suboptimal concentrations of anti-IL-4 receptor monoclonal antibodies. J Immunol. 1993; 150(2):394-398. (Biology). View Reference
-
Litton MJ, Sander B, Murphy E, O'Garra A, Abrams JS. Early expression of cytokines in lymph nodes after treatment in vivo with Staphylococcus enterotoxin B. J Immunol Methods. 1994; 175(1):47-58. (Biology). View Reference
-
Paul WE. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991; 77(9):1859-1870. (Biology). View Reference
-
Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995; 188(1):117-128. (Methodology: Flow cytometry). View Reference
-
Sadick MD, Heinzel FP, Holaday BJ, Pu RT, Dawkins RS, Locksley RM. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990; 171(1):115-127. (Biology). View Reference
-
Sander B, Hoiden I, Andersson U, Moller E, Abrams JS. Similar frequencies and kinetics of cytokine producing cells in murine peripheral blood and spleen. Cytokine detection by immunoassay and intracellular immunostaining. J Immunol Methods. 1993; 166(2):201-214. (Biology). View Reference
-
Shirai A, Sierra V, Kelly CI, Klinman DM. Individual cells simultaneously produce both IL-4 and IL-6 in vivo. Cytokine. 1994; 6(3):329-336. (Biology). View Reference
-
Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990; 145(11):3796-3806. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.