Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?




Flow cytometry analysis of Alexa Fluor® 647 Mouse Anti-Nanog on mouse embryonic stem (ES) cells (Left Panel). Mouse ES-E14TG2a (ATCC, Cat. No. CRL-1821) cells were fixed (BD Cytofix™ buffer, Cat. No. 554655) for 10 minutes at 37°C, permeabilized with BD™ Phosflow Perm Buffer III (Cat. No. 558050) on ice for 30 minutes, and then stained with either Alexa Fluor® 647 Mouse Anti-Nanog (solid line) or Alexa Fluor® 647 Mouse IgG1,k Isotype Control (Cat. No. 557732, dashed line). This antibody also works in BD™ Phosflow Perm Buffer I (Cat. No. 557885) and II (Cat. No. 558052). Flow cytometry was performed on a BD FACSCanto™ II flow cytometry system. Immunofluorescent staining of a mouse embryonic stem cell line (Right Panel). ES-E14TG2a cells (ATCC CRL-1821) were seeded in a 96-well imaging plate (Cat. No. 353219) at ~10,000 cells per well. After overnight incubation, the cells were fixed, permeabilized with Triton™ X-100, and stained with the Alexa Fluor® 647 Mouse Anti-Nanog antibody (pseudo colored magenta). Cell nuclei were counter-stained with Hoechst 33342 (pseudo colored blue). The images were captured on a BD Pathway™ 435 Cell Analyzer using a 20X objective and merged using BD AttoVision™ software. This antibody also stains F9 cells (mouse embryonal carcinoma, ATCC CRL-1720). It also worked with the Saponin and cold methanol fix/perm protocols, however Saponin permeabilization resulted in higher background staining.


BD Pharmingen™ Alexa Fluor® 647 Mouse anti-Mouse Nanog

监管状态图例
未经BD明确书面授权,严禁使用未经许可的任何商品。
准备和存储
推荐的实验流程
Bioimaging
1. Seed the cells in appropriate culture medium at an appropriate cell density in a BD Falcon™ 96-well Imaging Plate (Cat. No. 353219), and
culture overnight to 48 hours.
2. Remove the culture medium from the wells, and wash (one to two times) with 100 μl of 1× PBS.
3. Fix the cells by adding 100 µl of fresh 3.7% Formaldehyde in PBS or BD Cytofix™ fixation buffer (Cat. No. 554655) to each well and incubating for 10 minutes at room temperature (RT).
4. Remove the fixative from the wells, and wash the wells (one to two times) with 100 μl of 1× PBS.
5. Permeabilize the cells using either cold methanol (a), Triton™ X-100 (b), or Saponin (c):
a. Add 100 µl of -20°C 90% methanol or -20°C BD™ Phosflow Perm Buffer III (Cat. No. 558050) to each well and incubate for 5 minutes at RT.
b. Add 100 µl of 0.1% Triton™ X-100 to each well and incubate for 5 minutes at RT.
c. Add 100 µl of 1× Perm/Wash buffer (Cat. No. 554723) to each well and incubate for 15 to 30 minutes at RT. Continue to use 1× Perm/Wash buffer for all subsequent wash and dilutions steps.
6. Remove the permeabilization buffer from the wells, and wash one to two times with 100 μl of appropriate buffer (either 1× PBS or 1× Perm/Wash buffer, see step 5.c.).
7. Optional blocking step: Remove the wash buffers, and block the cells by adding 100 µl of blocking buffer BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656) or 3% FBS in appropriate dilution buffer to each well and incubating for 15 to 30 minutes at RT.
8. Dilute the antibody to its optimal working concentration in appropriate dilution buffer. Titrate antibodies and second-step reagents to determine the optimal concentration.
9. Add 50 µl of diluted antibody per well and incubate for 60 minutes at RT. Incubate in the dark if using fluorescently labeled antibodies.
10. Remove the antibody, and wash the wells three times with 100 μl of wash buffer. An optional detergent wash (100 μl of 0.05% Tween in 1× PBS) can be included prior to the regular wash steps.
11. If the antibody being used is fluorescently labeled, then move to step 12. Otherwise, if using a purified unlabeled antibody, repeat steps 8 to 10 with a fluorescently labeled second-step reagent to detect the purified antibody.
12. After the final wash, counter-stain the nuclei by adding 100 μl of a 2 μg/ml solution of Hoechst 33342 (eg, Sigma-Aldrich Cat. No. B2261) in 1× PBS to each well at least 15 minutes before imaging.
13. View and analyze the cells on an appropriate imaging instrument. Recommended filters for the BD Pathway™ instruments are:
Instrument Excitation Emission Dichroic
BD Pathway 855 620/60 700/75 660 LP
BD Pathway 435 628/40 690/40 FF660
商品通知
- This reagent has been pre-diluted for use at the recommended Volume per Test. We typically use 1 × 10^6 cells in a 100-µl experimental sample (a test).
- Alexa Fluor® 647 fluorochrome emission is collected at the same instrument settings as for allophycocyanin (APC).
- Alexa Fluor® is a registered trademark of Molecular Probes, Inc., Eugene, OR.
- The Alexa Fluor®, Pacific Blue™, and Cascade Blue® dye antibody conjugates in this product are sold under license from Molecular Probes, Inc. for research use only, excluding use in combination with microarrays, or as analyte specific reagents. The Alexa Fluor® dyes (except for Alexa Fluor® 430), Pacific Blue™ dye, and Cascade Blue® dye are covered by pending and issued patents.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
配套商品
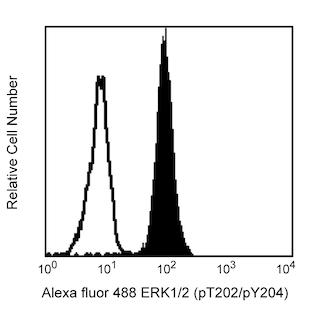


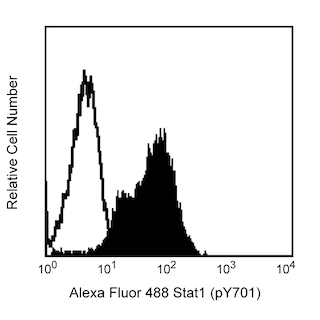

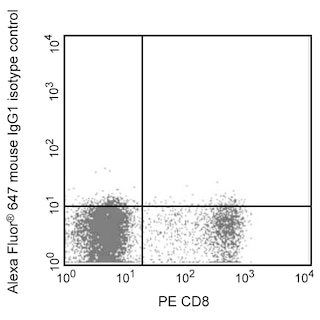
The M55-312 monoclonal antibody reacts with mouse Nanog (named for Tir Na Nog, the land of the ever-young of Celtic mythology), which is a homeobox transcription factor required for the maintenance of the undifferentiated state of pluripotent stem cells. Nanog expression counteracts the differentiation-promoting signals induced by the extrinsic factors LIF (Leukemia Inhibitory Factor) and BMP (Bone Morphogenic Protein). When Nanog expression is down-regulated, cell differentiation can proceed. Proteins that regulate Nanog expression include transcription factors Oct4, SOX2, FoxD3, and Tcf3 and tumor suppressor p53.
研发参考 (5)
-
Chambers I, Colby D, Robertson M, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003; 113:643-655. (Biology). 查看参考
-
Chambers I. The molecular basis of pluripotency in mouse embryonic stem cells. Cloning Stem Cells. 2004; 6(4):386-391. (Biology). 查看参考
-
Mitsui K, Tokuzawa Y, Itoh H, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003; 113:631-642. (Biology). 查看参考
-
Pan G, Thomson JA. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007; 17:42-49. (Biology). 查看参考
-
Sun Y, Li H, Yang H, Rao MS, Zhan M. Mechanisms controlling embryonic stem cell self-renewal and differentiation. Crit Rev Eukaryot Gene Expr.. 2006; 16(3):211-231. (Biology). 查看参考
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.